A novel pancreatic β-cell targeting bispecific-antibody (BsAb) can prevent the development of type 1 diabetes in NOD mice
- PMID: 24792135
- PMCID: PMC4077286
- DOI: 10.1016/j.clim.2014.04.014
A novel pancreatic β-cell targeting bispecific-antibody (BsAb) can prevent the development of type 1 diabetes in NOD mice
Abstract
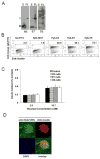
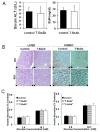
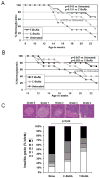
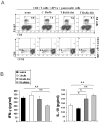
To prepare a novel Bispecific Antibody (BsAb) as a potential targeted therapy for T1D, we produced a "functionally inert" monoclonal antibody (mAb) against Glucose transporter-2 (GLUT-2) expressed on β-cells to serve as an anchoring antibody. The therapeutic arm is an agonistic mAb against Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4), a negative regulator of T-cell activation expressed on activated CD4+ T-cells. A BsAb was prepared by chemically coupling an anti-GLUT2 mAb to an agonistic anti-CTLA-4 mAb. This BsAb was able to bind to GLUT2 and CTLA-4 in vitro, and to pancreatic islets, both in vitro and in vivo. We tested the safety and efficacy of this BsAb by treating Non-Obese Diabetes (NOD) mice and found that it could delay the onset of diabetes with no apparent undesirable side effects. Thus, engagement of CTLA-4 on activated T cells from target tissue can be an effective way to treat type-1 diabetes.
Keywords: Anti-CTLA-4; Anti-Glut2; Dendritic cells; Diabetes; Regulatory T cells; Tolerance.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Dendritic cell-directed CTLA-4 engagement during pancreatic beta cell antigen presentation delays type 1 diabetes.J Immunol. 2010 Jun 15;184(12):6695-708. doi: 10.4049/jimmunol.0903130. Epub 2010 May 14. J Immunol. 2010. PMID: 20483724 Free PMC article.
-
Transgenic expression of single-chain anti-CTLA-4 Fv on beta cells protects nonobese diabetic mice from autoimmune diabetes.J Immunol. 2009 Aug 15;183(4):2277-85. doi: 10.4049/jimmunol.0900679. Epub 2009 Jul 27. J Immunol. 2009. PMID: 19635924
-
A bispecific protein capable of engaging CTLA-4 and MHCII protects non-obese diabetic mice from autoimmune diabetes.PLoS One. 2013 May 21;8(5):e63530. doi: 10.1371/journal.pone.0063530. Print 2013. PLoS One. 2013. PMID: 23704916 Free PMC article.
-
Immunotherapy for the prevention and treatment of type 1 diabetes.Int Rev Immunol. 2005 Sep-Dec;24(5-6):307-26. doi: 10.1080/08830180500379721. Int Rev Immunol. 2005. PMID: 16318984 Review.
-
Immunotherapy of type 1 diabetes.Arch Immunol Ther Exp (Warsz). 2008 Jul-Aug;56(4):227-36. doi: 10.1007/s00005-008-0025-2. Epub 2008 Jul 29. Arch Immunol Ther Exp (Warsz). 2008. PMID: 18726144 Review.
Cited by
-
A Multiple Antigenic Peptide Mimicking Peptidoglycan Induced T Cell Responses to Protect Mice from Systemic Infection with Staphylococcus aureus.PLoS One. 2015 Aug 28;10(8):e0136888. doi: 10.1371/journal.pone.0136888. eCollection 2015. PLoS One. 2015. PMID: 26317210 Free PMC article.
-
Review on Monoclonal Antibodies (mAbs) as a Therapeutic Approach for Type 1 Diabetes.Curr Diabetes Rev. 2024;20(7):e310823220578. doi: 10.2174/1573399820666230831153249. Curr Diabetes Rev. 2024. PMID: 37653635 Review.
-
The role of IL-25 in the reduction of oxidative stress and the apoptosis of airway epithelial cells with specific immunotherapy in an asthma mouse model.Am J Transl Res. 2017 Sep 15;9(9):4137-4148. eCollection 2017. Am J Transl Res. 2017. PMID: 28979688 Free PMC article.
-
Orally-Induced Intestinal CD4+ CD25+ FoxP3+ Treg Controlled Undesired Responses towards Oral Antigens and Effectively Dampened Food Allergic Reactions.PLoS One. 2015 Oct 30;10(10):e0141116. doi: 10.1371/journal.pone.0141116. eCollection 2015. PLoS One. 2015. PMID: 26517875 Free PMC article.
-
Effects of Bifidobacterium Breve Feeding Strategy and Delivery Modes on Experimental Allergic Rhinitis Mice.PLoS One. 2015 Oct 7;10(10):e0140018. doi: 10.1371/journal.pone.0140018. eCollection 2015. PLoS One. 2015. PMID: 26445348 Free PMC article.
References
-
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358(9277):221–9. - PubMed
-
- Todd JA, Wicker LS. Genetic protection from the inflammatory disease type 1 diabetes in humans and animal models. Immunity. 2001;15(3):387–95. - PubMed
-
- Yoon JW, Jun HS. Cellular and molecular pathogenic mechanisms of insulin-dependent diabetes mellitus. Ann N Y Acad Sci. 2001;928:200–11. - PubMed
-
- Skarsvik S, et al. Poor in vitro maturation and pro-inflammatory cytokine response of dendritic cells in children at genetic risk of type 1 diabetes. Scand J Immunol. 2004;60 (6):647–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

