Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration
- PMID: 24792589
- PMCID: PMC4122592
- DOI: 10.1016/j.exer.2014.04.015
Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration
Abstract
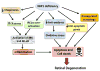
The endoplasmic reticulum (ER) is the primary intracellular organelle responsible for protein and lipid biosynthesis, protein folding and trafficking, calcium homeostasis, and several other vital processes in cell physiology. Disturbance in ER function results in ER stress and subsequent activation of the unfolded protein response (UPR). The UPR up-regulates ER chaperones, reduces protein translation, and promotes clearance of cytotoxic misfolded proteins to restore ER homeostasis. If this vital process fails, the cell will be signaled to enter apoptosis, resulting in cell death. Sustained ER stress also can trigger an inflammatory response and exacerbate oxidative stress, both of which contribute synergistically to tissue damage. Studies performed over the past decade have implicated ER stress in a broad range of human diseases, including neurodegenerative diseases, cancer, diabetes, and vascular disorders. Several of these diseases also entail retinal dysfunction and degeneration caused by injury to retinal neurons and/or to the blood vessels that supply retinal cells with nutrients, trophic and homeostatic factors, oxygen, and other essential molecules, as well as serving as a conduit for removal of waste products and potentially toxic substances from the retina. Collectively, such injuries represent the leading cause of blindness world-wide in all age groups. Herein, we summarize recent progress on the study of ER stress and UPR signaling in retinal biology and discuss the molecular mechanisms and the potential clinical applications of targeting ER stress as a new therapeutic approach to prevent and treat neuronal degeneration in the retina.
Keywords: apoptosis; cell death; endoplasmic reticulum stress; inflammation; retinal degeneration; unfolded protein response.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
-
- Adachi T, et al. Endoplasmic reticulum stress induces retinal endothelial permeability of extracellular-superoxide dismutase. Free Radical Research. 2011;45:1083–1092. - PubMed
-
- Alberts B, et al. Molecular biology of the cell. Garland Science; New York: 2002.
-
- Allingham RR, et al. Shields Textbook of Glaucoma. 6. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2010. p. 656.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

