Extracellular matrix-regulated neural differentiation of human multipotent marrow progenitor cells enhances functional recovery after spinal cord injury
- PMID: 24792783
- PMCID: PMC4692164
- DOI: 10.1016/j.spinee.2014.04.024
Extracellular matrix-regulated neural differentiation of human multipotent marrow progenitor cells enhances functional recovery after spinal cord injury
Abstract
Background context: Recent advanced studies have demonstrated that cytokines and extracellular matrix (ECM) could trigger various types of neural differentiation. However, the efficacy of differentiation and in vivo transplantation has not yet thoroughly been investigated.
Purpose: To highlight the current understanding of the effects of ECM on neural differentiation of human bone marrow-derived multipotent progenitor cells (MPCs), regarding state-of-art cure for the animal with acute spinal cord injury (SCI), and explore future treatments aimed at neural repair.
Study design: A selective overview of the literature pertaining to the neural differentiation of the MSCs and experimental animals aimed at improved repair of SCI.
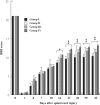
Methods: Extracellular matrix proteins, tenascin-cytotactin (TN-C), tenascin-restrictin (TN-R), and chondroitin sulfate (CS), with the cytokines, nerve growth factor (NGF)/brain-derived neurotrophic factor (BDNF)/retinoic acid (RA) (NBR), were incorporated to induce transdifferentiation of human MPCs. Cells were treated with NBR for 7 days, and then TN-C, TN-R, or CS was added for 2 days. The medium was changed every 2 days. Twenty-four animals were randomly assigned to four groups with six animals in each group: one experimental and three controls. Animals received two (bilateral) injections of vehicle, MPCs, NBR-induced MPCs, or NBR/TN-C-induced MPCs into the lesion sites after SCI. Functional assessment was measured using the Basso, Beattie, and Bresnahan locomotor rating score. Data were analyzed using analysis of variance followed by Student-Newman-Keuls (SNK) post hoc tests.
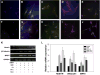
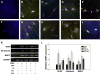
Results: Results showed that MPCs with the transdifferentiation of human MPCs to neurons were associated with increased messenger-RNA (mRNA) expression of neuronal markers including nestin, microtubule-associated protein (MAP) 2, glial fibrillary acidic protein, βIII tubulin, and NGF. Greater amounts of neuronal morphology appeared in cultures incorporated with TN-C and TN-R than those with CS. The addition of TN-C enhanced mRNA expressions of MAP2, βIII tubulin, and NGF, whereas TN-R did not significantly change. Conversely, CS exposure decreased MAP2, βIII tubulin, and NGF expressions. The TN-C-treated MSCs significantly and functionally repaired SCI-induced rats at Day 42. Present results indicate that ECM components, such as tenascins and CS in addition to cytokines, may play functional roles in regulating neurogenesis by human MPCs.
Conclusions: These findings suggest that the combined use of TN-C, NBR, and human MPCs offers a new feasible method for nerve repair.
Keywords: Chondroitin sulfate; Extracellular matrix; Human multipotent progenitor cells; Neurogenesis; Tenascin-cytotactin; Tenascin-restrictin.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Effects of combination treatment with transcranial magnetic stimulation and bone marrow mesenchymal stem cell transplantation or Raf inhibition on spinal cord injury in rats.Mol Med Rep. 2021 Apr;23(4):294. doi: 10.3892/mmr.2021.11934. Epub 2021 Mar 2. Mol Med Rep. 2021. PMID: 33649786 Free PMC article.
-
Effects of Edaravone on Functional Recovery of a Rat Model with Spinal Cord Injury Through Induced Differentiation of Bone Marrow Mesenchymal Stem Cells into Neuron-Like Cells.Cell Reprogram. 2021 Feb;23(1):47-56. doi: 10.1089/cell.2020.0055. Epub 2021 Jan 5. Cell Reprogram. 2021. PMID: 33400610
-
Bone marrow-derived mesenchymal stem cell transplantation for chronic spinal cord injury in rats: comparative study between intralesional and intravenous transplantation.Spine (Phila Pa 1976). 2013 Aug 1;38(17):E1065-74. doi: 10.1097/BRS.0b013e31829839fa. Spine (Phila Pa 1976). 2013. PMID: 23629485
-
A combination of mesenchymal stem cells and scaffolds promotes motor functional recovery in spinal cord injury: a systematic review and meta-analysis.J Neurosurg Spine. 2019 Nov 1;32(2):269-284. doi: 10.3171/2019.8.SPINE19201. Print 2020 Feb 1. J Neurosurg Spine. 2019. PMID: 31675724
-
Extracellular Matrix and Oxidative Stress Following Traumatic Spinal Cord Injury: Physiological and Pathophysiological Roles and Opportunities for Therapeutic Intervention.Antioxid Redox Signal. 2022 Jul;37(1-3):184-207. doi: 10.1089/ars.2021.0120. Epub 2021 Dec 7. Antioxid Redox Signal. 2022. PMID: 34465134 Review.
Cited by
-
Laminin and Platelet-Derived Growth Factor-BB Promote Neuronal Differentiation of Human Urine-Derived Stem Cells.Tissue Eng Regen Med. 2017 Dec 28;15(2):195-209. doi: 10.1007/s13770-017-0102-x. eCollection 2018 Apr. Tissue Eng Regen Med. 2017. PMID: 30603547 Free PMC article.
-
Effect of a Bone Marrow-Derived Extracellular Matrix on Cell Adhesion and Neural Induction of Dental Pulp Stem Cells.Front Cell Dev Biol. 2020 Mar 6;8:100. doi: 10.3389/fcell.2020.00100. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32211401 Free PMC article.
References
-
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. - PubMed
-
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nature Neuroscience. 2002;5:438–45. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. - PubMed
-
- Chen J, Li Y, Wang L, et al. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

