Human airway smooth muscle maintain in situ cell orientation and phenotype when cultured on aligned electrospun scaffolds
- PMID: 24793171
- PMCID: PMC4080283
- DOI: 10.1152/ajplung.00318.2013
Human airway smooth muscle maintain in situ cell orientation and phenotype when cultured on aligned electrospun scaffolds
Erratum in
-
Corrigendum. Human airway smooth muscle maintain in situ cell orientation and phenotype when cultured on aligned electrospun scaffolds.Am J Physiol Lung Cell Mol Physiol. 2015 Apr 1;308(7):L729. doi: 10.1152/ajplung.zh5-6728-corr.2015. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25833917 Free PMC article. No abstract available.
Abstract
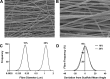
Human airway smooth muscle (HASM) contraction plays a central role in regulating airway resistance in both healthy and asthmatic bronchioles. In vitro studies that investigate the intricate mechanisms that regulate this contractile process are predominantly conducted on tissue culture plastic, a rigid, 2D geometry, unlike the 3D microenvironment smooth muscle cells are exposed to in situ. It is increasingly apparent that cellular characteristics and responses are altered between cells cultured on 2D substrates compared with 3D topographies. Electrospinning is an attractive method to produce 3D topographies for cell culturing as the fibers produced have dimensions within the nanometer range, similar to cells' natural environment. We have developed an electrospun scaffold using the nondegradable, nontoxic, polymer polyethylene terephthalate (PET) composed of uniaxially orientated nanofibers and have evaluated this topography's effect on HASM cell adhesion, alignment, and morphology. The fibers orientation provided contact guidance enabling the formation of fully aligned sheets of smooth muscle. Moreover, smooth muscle cells cultured on the scaffold present an elongated cell phenotype with altered contractile protein levels and distribution. HASM cells cultured on this scaffold responded to the bronchoconstrictor bradykinin. The platform presented provides a novel in vitro model that promotes airway smooth muscle cell development toward a more in vivo-like phenotype while providing topological cues to ensure full cell alignment.
Keywords: airway smooth muscle; aligned fibers; electrospinning; in vitro model; tissue engineering.
Copyright © 2014 the American Physiological Society.
Figures






References
-
- Ahmed I, Ponery AS, Nur EKA, Kamal J, Meshel AS, Sheetz MP, Schindler M, Meiners S. Morphology, cytoskeletal organization, and myosin dynamics of mouse embryonic fibroblasts cultured on nanofibrillar surfaces. Mol Cell Biochem 301: 241–249, 2007. - PubMed
-
- Ahvaz HH, Soleimani M, Mobasheri H, Bakhshandeh B, Shakhssalim N, Soudi S, Hafizi M, Vasei M, Dodel M. Effective combination of hydrostatic pressure and aligned nanofibrous scaffolds on human bladder smooth muscle cells: implication for bladder tissue engineering. J Mater Sci Mater Med 23: 2281–2290, 2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

