The long elusive IgM Fc receptor, FcμR
- PMID: 24793544
- PMCID: PMC4160156
- DOI: 10.1007/s10875-014-0022-7
The long elusive IgM Fc receptor, FcμR
Abstract
IgM exists as both a monomer on the surface of B cells and a pentamer secreted by plasma cells. Both pre-immune "natural" and antigen-induced "immune" IgM antibodies are important for protective immunity and for immune regulation of autoimmune processes by recognizing pathogens and self-antigens. Effector proteins interacting with the Fc portion of IgM, such as complement and complement receptors, have thus far been proposed but fail to fully account for the IgM-mediated protection and regulation. A major reason for this deficit in our understanding of IgM function seems to be lack of data on a long elusive Fc receptor for IgM (FcμR). We have recently identified a bona fide FcμR in both humans and mice. In this article we briefly review what we have learned so far about FcμR.
Conflict of interest statement
Figures
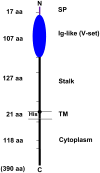
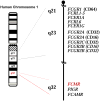

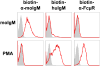



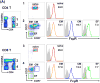

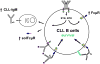
References
-
- Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–92. - PubMed
-
- Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. - PubMed
-
- Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. - PubMed
-
- Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005 Jul;23(1):41–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

