Aminoadamantanes with persistent in vitro efficacy against H1N1 (2009) influenza A
- PMID: 24793875
- PMCID: PMC4127532
- DOI: 10.1021/jm500598u
Aminoadamantanes with persistent in vitro efficacy against H1N1 (2009) influenza A
Abstract
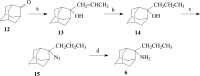
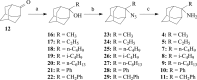
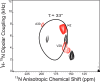
A series of 2-adamantanamines with alkyl adducts of various lengths were examined for efficacy against strains of influenza A including those having an S31N mutation in M2 proton channel that confer resistance to amantadine and rimantadine. The addition of as little as one CH2 group to the methyl adduct of the amantadine/rimantadine analogue, 2-methyl-2-aminoadamantane, led to activity in vitro against two M2 S31N viruses A/Calif/07/2009 (H1N1) and A/PR/8/34 (H1N1) but not to a third A/WS/33 (H1N1). Solid state NMR of the transmembrane domain (TMD) with a site mutation corresponding to S31N shows evidence of drug binding. But electrophysiology using the full length S31N M2 protein in HEK cells showed no blockade. A wild type strain, A/Hong Kong/1/68 (H3N2) developed resistance to representative drugs within one passage with mutations in M2 TMD, but A/Calif/07/2009 S31N was slow (>8 passages) to develop resistance in vitro, and the resistant virus had no mutations in M2 TMD. The results indicate that 2-alkyl-2-aminoadamantane derivatives with sufficient adducts can persistently block p2009 influenza A in vitro through an alternative mechanism. The observations of an HA1 mutation, N160D, near the sialic acid binding site in both 6-resistant A/Calif/07/2009(H1N1) and the broadly resistant A/WS/33(H1N1) and of an HA1 mutation, I325S, in the 6-resistant virus at a cell-culture stable site suggest that the drugs tested here may block infection by direct binding near these critical sites for virus entry to the host cell.
Figures
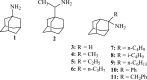

References
-
- Bright R. A.; Medina M. J.; Xu X.; Perez-Oronoz G.; Wallis T. R.; Davis X. M.; Povinelli L.; Cox N. J.; Klimov A. I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 2005, 366, 1175–1181. - PubMed
-
- Bright R. A.; Shay D. K.; Shu B.; Cox N. J.; Klimov A. I. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA, J. Am. Med. Assoc. 2006, 295, 891–894. - PubMed
- Lan Y.; Zhang Y.; Dong L.; Wang D.; Huang W.; Xin L.; Yang L.; Zhao X.; Li Z.; Wang W.; Li X.; Xu C.; Guo J.; Wang M.; Peng Y.; Gao Y.; Guo Y.; Wen L.; Jiang T.; Shu Y. A comprehensive surveillance of adamantane resistance among human influenza A virus isolated from mainland China between 1956 and 2009. Antiviral Ther. 2010, 15, 853–859. - PubMed
-
- High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents—United States, 2005–06 influenza season. Morbidity Mortality Wkly. Rep. 2006, 55, 44–46. - PubMed
-
-
See, for examples, the following:
- Kolocouris N.; Foscolos G. B.; Kolocouris A.; Marakos P.; Pouli N.; Fytas G.; Ikeda S.; De Clercq E. Synthesis and antiviral activity evaluation of some aminoadamantane derivatives. J. Med. Chem. 1994, 37, 2896–2902. - PubMed
- Kolocouris A.; Spearpoint P.; Martin S. R.; Hay A. J.; Lopez-Querol M.; Sureda F. X.; Padalko E.; Neyts J.; De Clercq E. Comparisons of the influenza virus A M2 channel binding affinities, anti-influenza virus potencies and NMDA antagonistic activities of 2-alkyl-2-aminoadamantanes and analogues. Bioorg. Med. Chem. Lett. 2008, 18, 6156–6160. - PubMed
-
-
- Stouffer A. L.; Acharya R.; Salom D.; Levine A. S.; Di Costanzo L.; Soto C. S.; Tereshko V.; Nanda V.; Stayrook S.; DeGrado W. F. Structural basis for the function and inhibition of an influenza virus proton channel. Nature 2008, 451, 596–599. - PMC - PubMed
- Cady S. D.; Schmidt-Rohr K.; Wang J.; Soto C. S.; Degrado W. F.; Hong M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature 2010, 463, 689–692. - PMC - PubMed
- Cady S. D.; Wang J.; Wu Y.; DeGrado W. F.; Hong M. Specific binding of adamantane drugs and direction of their polar amines in the pore of the influenza M2 transmembrane domain in lipid bilayers and dodecylphosphocholine micelles determined by NMR spectroscopy. J. Am. Chem. Soc. 2011, 133, 4274–4284. - PMC - PubMed
- Pielak R. M.; Oxenoid K.; Chou J. J. Structural investigation of rimantadine inhibition of the AM2-BM2 chimera channel of influenza viruses. Structure 2011, 19, 1655–1663. - PMC - PubMed
- Hu J.; Fu R.; Cross T. A. The chemical and dynamical influence of the anti-viral drug amantadine on the M2 proton channel transmembrane domain. Biophys. J. 2007, 93, 276–283. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

