Endothelial and leukocyte heparan sulfates regulate the development of allergen-induced airway remodeling in a mouse model
- PMID: 24794009
- PMCID: PMC4070979
- DOI: 10.1093/glycob/cwu035
Endothelial and leukocyte heparan sulfates regulate the development of allergen-induced airway remodeling in a mouse model
Abstract
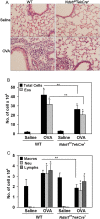
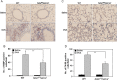
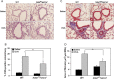
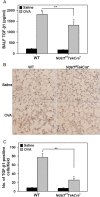
Heparan sulfate (HS) proteoglycans (HSPGs) participate in several aspects of inflammation because of their ability to bind to growth factors, chemokines, interleukins and extracellular matrix proteins as well as promote inflammatory cell trafficking and migration. We investigated whether HSPGs play a role in the development of airway remodeling during chronic allergic asthma using mice deficient in endothelial- and leukocyte-expressed N-deacetylase/N-sulfotransferase-1 (Ndst1), an enzyme involved in modification reactions during HS biosynthesis. Ndst1-deficient and wild-type (WT) mice exposed to repetitive allergen (ovalbumin [OVA]) challenge were evaluated for the development of airway remodeling. Chronic OVA-challenged WT mice exhibited increased HS expression in the lungs along with airway eosinophilia, mucus hypersecretion, peribronchial fibrosis, increased airway epithelial thickness and smooth muscle mass. In OVA-challenged Ndst1-deficient mice, lung eosinophil and macrophage infiltration as well as airway mucus accumulation, peribronchial fibrosis and airway epithelial thickness were significantly lower than in allergen-challenged WT mice along with a trend toward decreased airway smooth muscle mass. Leukocyte and endothelial Ndst 1 deficiency also resulted in significantly decreased expression of IL-13 as well as remodeling-associated mediators such as VEGF, FGF-2 and TGF-β1 in the lung tissue. At a cellular level, exposure to eotaxin-1 failed to induce TGF-β1 expression by Ndst1-deficient eosinophils relative to WT eosinophils. These studies suggest that leukocyte and endothelial Ndst1-modified HS contribute to the development of allergen-induced airway remodeling by promoting recruitment of inflammatory cells as well as regulating expression of pro-remodeling factors such as IL-13, VEGF, TGF-β1 and FGF-2 in the lung.
Keywords: FGF-2; N-Deacetylase/N-Sulfotransferase-1; TGF-β; allergen-induced airway remodeling; heparan sulfates.
© The Author 2014. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




Similar articles
-
Regulation of eosinophil recruitment and allergic airway inflammation by heparan sulfate proteoglycan (HSPG) modifying enzymes.Exp Lung Res. 2018 Mar;44(2):98-112. doi: 10.1080/01902148.2018.1451574. Epub 2018 Apr 5. Exp Lung Res. 2018. PMID: 29621420 Free PMC article.
-
Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation.J Immunol. 2009 Sep 15;183(6):3971-9. doi: 10.4049/jimmunol.0901604. Epub 2009 Aug 26. J Immunol. 2009. PMID: 19710461 Free PMC article.
-
Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13.Respir Res. 2010 Nov 1;11(1):154. doi: 10.1186/1465-9921-11-154. Respir Res. 2010. PMID: 21040544 Free PMC article.
-
Cytokines and growth factors in airway remodeling in asthma.Curr Opin Immunol. 2007 Dec;19(6):676-80. doi: 10.1016/j.coi.2007.07.017. Epub 2007 Aug 27. Curr Opin Immunol. 2007. PMID: 17720466 Review.
-
Macrophages Orchestrate Airway Inflammation, Remodeling, and Resolution in Asthma.Int J Mol Sci. 2023 Jun 21;24(13):10451. doi: 10.3390/ijms241310451. Int J Mol Sci. 2023. PMID: 37445635 Free PMC article. Review.
Cited by
-
Endothelial heparan sulfate deficiency reduces inflammation and fibrosis in murine diabetic nephropathy.Lab Invest. 2018 Apr;98(4):427-438. doi: 10.1038/s41374-017-0015-2. Epub 2018 Jan 12. Lab Invest. 2018. PMID: 29330473 Free PMC article.
-
Regulation of eosinophil recruitment and allergic airway inflammation by heparan sulfate proteoglycan (HSPG) modifying enzymes.Exp Lung Res. 2018 Mar;44(2):98-112. doi: 10.1080/01902148.2018.1451574. Epub 2018 Apr 5. Exp Lung Res. 2018. PMID: 29621420 Free PMC article.
-
Physiology and Pathophysiology of Heparan Sulfate in Animal Models: Its Biosynthesis and Degradation.Int J Mol Sci. 2022 Feb 10;23(4):1963. doi: 10.3390/ijms23041963. Int J Mol Sci. 2022. PMID: 35216081 Free PMC article. Review.
-
Selective Deletion of Heparan Sulfotransferase Enzyme, Ndst1, in Donor Endothelial and Myeloid Precursor Cells Significantly Decreases Acute Allograft Rejection.Sci Rep. 2018 Sep 7;8(1):13433. doi: 10.1038/s41598-018-31779-7. Sci Rep. 2018. PMID: 30194334 Free PMC article.
-
Effect of Polarization and Chronic Inflammation on Macrophage Expression of Heparan Sulfate Proteoglycans and Biosynthesis Enzymes.J Histochem Cytochem. 2019 Jan;67(1):9-27. doi: 10.1369/0022155418798770. Epub 2018 Sep 11. J Histochem Cytochem. 2019. PMID: 30205019 Free PMC article. Review.
References
-
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42.
-
- Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–462. - PubMed
-
- Ali S, Fritchley SJ, Chaffey BT, Kirby JA. Contribution of the putative heparan sulfate-binding motif BBXB of RANTES to transendothelial migration. Glycobiology. 2002;12:535–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

