Calcium-dependent PKC isoforms have specialized roles in short-term synaptic plasticity
- PMID: 24794094
- PMCID: PMC4097165
- DOI: 10.1016/j.neuron.2014.04.003
Calcium-dependent PKC isoforms have specialized roles in short-term synaptic plasticity
Abstract
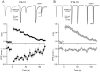
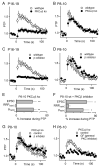
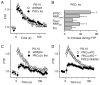
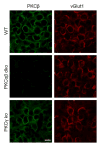
Posttetanic potentiation (PTP) is a widely observed form of short-term plasticity lasting for tens of seconds after high-frequency stimulation. Here we show that although protein kinase C (PKC) mediates PTP at the calyx of Held synapse in the auditory brainstem before and after hearing onset, PTP is produced primarily by an increased probability of release (p) before hearing onset, and by an increased readily releasable pool of vesicles (RRP) thereafter. We find that these mechanistic differences, which have distinct functional consequences, reflect unexpected differential actions of closely related calcium-dependent PKC isoforms. Prior to hearing onset, when PKCγ and PKCβ are both present, PKCγ mediates PTP by increasing p and partially suppressing PKCβ actions. After hearing onset, PKCγ is absent and PKCβ produces PTP by increasing RRP. In hearing animals, virally expressed PKCγ overrides PKCβ to produce PTP by increasing p. Thus, two similar PKC isoforms mediate PTP in distinctly different ways.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–1262. - PubMed
-
- Bao JX, Kandel ER, Hawkins RD. Involvement of pre- and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science. 1997;275:969–973. - PubMed
-
- Barclay JW, Craig TJ, Fisher RJ, Ciufo LF, Evans GJ, Morgan A, Burgoyne RD. Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. J Biol Chem. 2003;278:10538–10545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

