Elution of High-affinity (>10-9 KD) Antibodies from Tissue Sections: Clues to the Molecular Mechanism and Use in Sequential Immunostaining
- PMID: 24794148
- PMCID: PMC4174624
- DOI: 10.1369/0022155414536732
Elution of High-affinity (>10-9 KD) Antibodies from Tissue Sections: Clues to the Molecular Mechanism and Use in Sequential Immunostaining
Abstract
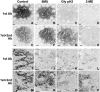
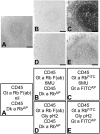
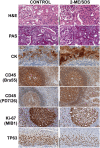
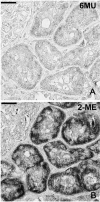
Inconsistent results obtained with published methods for the elution of antibodies from tissue sections prompted the assessment of both old and new methods in combination with monoclonal rabbit antibodies of known, increased affinity (above 1×10(-9) KD). We tested an acidic (pH 2) glycine buffer, a 6 M urea hot buffer and a 2-Mercaptoethanol, SDS buffer (2-ME/SDS). Some antibodies were not removed by the glycine pH 2 or 6 M urea hot buffers, indicating that antibodies survive much harsher conditions than previously believed. We found that the elution is dependent upon the antibody affinity and is reduced by species-specific crosslinking via a dimeric or Fab fragments of a secondary antibody. The high affinity bond of exogenous streptavidin with the endogenous biotin can be removed by 6 M urea but not by the other buffers. 2-ME/SDS buffer is superior to glycine pH 2 and 6 M urea hot elution buffers for all antibodies because of its irreversible effect on the structure of the antibodies. It also has a mild retrieving effect on some antigens present on routinely treated sections and no detrimental effect on the immunoreactivity of the tissue. Therefore, 2-ME/SDS buffer is the method of choice to perform multiple rounds of immunostaining on a single routine section.
Keywords: affinity; antibody; elution; multiple immunostaining; stripping.
© The Author(s) 2014.
Conflict of interest statement
Figures

Similar articles
-
Mechanisms of heat-induced antigen retrieval: does pH or ionic strength of the solution play a role for refolding antigens?J Histochem Cytochem. 2005 Nov;53(11):1311-21. doi: 10.1369/jhc.5C6627.2005. Epub 2005 Jul 11. J Histochem Cytochem. 2005. PMID: 16009962
-
Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies.J Histochem Cytochem. 1995 Feb;43(2):193-201. doi: 10.1177/43.2.7822775. J Histochem Cytochem. 1995. PMID: 7822775
-
Isolation of specific IgM monoclonal antibodies by affinity chromatography using alkaline buffers.Mol Immunol. 1987 Jan;24(1):11-5. doi: 10.1016/0161-5890(87)90106-4. Mol Immunol. 1987. PMID: 3614204
-
Microwave antigen retrieval in immunocytochemistry: a study of 80 antibodies.J Clin Pathol. 1994 May;47(5):448-52. doi: 10.1136/jcp.47.5.448. J Clin Pathol. 1994. PMID: 7517960 Free PMC article.
-
A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections.J Immunol Methods. 2005 Apr;299(1-2):139-51. doi: 10.1016/j.jim.2005.02.006. Epub 2005 Apr 1. J Immunol Methods. 2005. PMID: 15896802
Cited by
-
Single-cell spatial proteomics.Histol Histopathol. 2025 Aug;40(8):1173-1184. doi: 10.14670/HH-18-861. Epub 2024 Dec 13. Histol Histopathol. 2025. PMID: 39744823 Review.
-
Characterisation of parasympathetic ascending nerves in human colon.Front Neurosci. 2022 Dec 1;16:1072002. doi: 10.3389/fnins.2022.1072002. eCollection 2022. Front Neurosci. 2022. PMID: 36532291 Free PMC article.
-
Antibody elution with 2-me/SDS solution: Uses for multi-layer immunohistochemical analysis of wholemount preparations of human colonic myenteric plexus.Heliyon. 2024 Feb 21;10(5):e26522. doi: 10.1016/j.heliyon.2024.e26522. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38434276 Free PMC article.
-
Epitope recognition in the human-pig comparison model on fixed and embedded material.J Histochem Cytochem. 2015 Oct;63(10):805-22. doi: 10.1369/0022155415597738. Epub 2015 Jul 24. J Histochem Cytochem. 2015. PMID: 26209082 Free PMC article.
-
A Simple Method for Creating a High-Content Microscope for Imaging Multiplexed Tissue Microarrays.Curr Protoc. 2021 Apr;1(4):e68. doi: 10.1002/cpz1.68. Curr Protoc. 2021. PMID: 33822482 Free PMC article.
References
-
- Airenne KJ, Marjomäki VS, Kulomaa MS. (1999). Recombinant avidin and avidin-fusion proteins. Biomol Eng 16:87-92 - PubMed
-
- Argentieri MC, Pilla D, Vanzati A, Lonardi S, Facchetti F, Doglioni C, Parravicini C, Cattoretti G. (2013). Antibodies are forever: a study using 12-26-year-old expired antibodies. Histopathology 63:869-876 - PubMed
-
- Bauer M, Schilling N, Spanel-Borowski K. (2001). Limitation of microwave treatment for double immunolabelling with antibodies of the same species and isotype. Histochem Cell Biol 116:227-232 - PubMed
-
- Brouns I, Van Nassauw L, Van Genechten J, Majewski M, Scheuermann DW, Timmermans J-P, Adriaensen D. (2002). Triple immunofluorescence staining with antibodies raised in the same species to study the complex innervation pattern of intrapulmonary chemoreceptors. J Histochem Cytochem 50:575-582 - PubMed
-
- Buscone S, Argentieri MC, Pilla D, Cattoretti G. (2014) Whole-slide, Quadruple Immunofluorescence Labeling of Routinely Processed Paraffin Sections. Appl immunohistochem Mol Morphol 22:e1-7 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

