Measures of outcome in metastatic breast cancer: insights from a real-world scenario
- PMID: 24794159
- PMCID: PMC4041678
- DOI: 10.1634/theoncologist.2014-0002
Measures of outcome in metastatic breast cancer: insights from a real-world scenario
Abstract
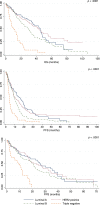
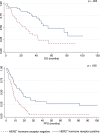
No gold standard treatment exists for metastatic breast cancer (MBC). Clinical decision making is based on knowledge of prognostic and predictive factors that are extrapolated from clinical trials and, sometimes, are not reliably transferable to a real-world scenario. Moreover, misalignment between endpoints used in drug development and measures of outcome in clinical practice has been noted. The roles of overall survival (OS) and progression-free survival (PFS) as primary endpoints in the context of clinical trials are the subjects of lively debate. Information about these parameters in routine clinical practice is potentially useful to design new studies and/or to interpret the results of clinical research. This study analyzed the impact of patient and tumor characteristics on the major measures of outcome across different lines of treatment in a cohort of 472 patients treated for MBC. OS, PFS, and postprogression survival (PPS) were analyzed. The study showed how biological and clinical characteristics may have different prognostic value across different lines of therapy for MBC. After first-line treatment, the median PPS of luminal A, luminal B, and human epidermal growth factor receptor 2 (HER2)-positive groups was longer than 12 months. The choice of OS as a primary endpoint for clinical trials could not be appropriate with these subtypes. In contrast, OS could be an appropriate endpoint when PPS is expected to be low (e.g., triple-negative subtype after the first line; other subtypes after the third line). The potential implications of these findings are clinical and methodological.
Keywords: Breast neoplasms; Outcome measure; Surrogate endpoint; Survival analysis.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. - PubMed
-
- O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. The Oncologist. 2005;10(suppl 3):20–29. - PubMed
-
- Greenberg PA, Hortobagyi GN, Smith TL, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol. 1996;14:2197–2205. - PubMed
-
- Verma S, Clemons M. First-line treatment options for patients with HER-2 negative metastatic breast cancer: The impact of modern adjuvant chemotherapy. The Oncologist. 2007;12:785–797. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

