Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment
- PMID: 24794217
- PMCID: PMC4008589
- DOI: 10.1371/journal.pone.0096565
Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment
Abstract
Objective: The immune response to pancreatic ductal adenocarcinoma (PDA) may play a role in defining its uniquely aggressive biology; therefore, we sought to clearly define the adaptive immune infiltrate in PDA.
Design: We used immunohistochemistry and flow cytometry to characterize the immune infiltrate in human PDA and compared our findings to the patients' peripheral blood.
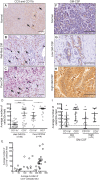
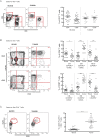
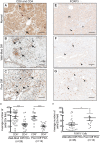
Results: In contrast to the myeloid cell predominant infiltrate seen in murine models, T cells comprised the majority of the hematopoietic cell component of the tumor stroma in human PDA. Most intratumoral CD8+ T cells exhibited an antigen-experienced effector memory cell phenotype and were capable of producing IFN-γ. CD4+ regulatory T cells (Treg) and IL-17 producing T helper cells were significantly more prevalent in tumor than in blood. Consistent with the association with reduced survival in previous studies, we observed higher frequencies of both myeloid cells and Treg in poorly differentiated tumors. The majority of intratumoral T cells expressed the co-inhibitory receptor programmed death-1 (PD-1), suggesting one potential mechanism through which PDA may evade antitumor immunity. Successful multimodal neoadjuvant therapy altered the immunoregulatory balance and was associated with reduced infiltration of both myeloid cells and Treg.
Conclusion: Our data show that human PDA contains a complex mixture of inflammatory and regulatory immune cells, and that neoadjuvant therapy attenuates the infiltration of intratumoral cells associated with immunosuppression and worsened survival.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

