Ecdysone mediates the development of immunity in the Drosophila embryo
- PMID: 24794300
- PMCID: PMC4030305
- DOI: 10.1016/j.cub.2014.03.062
Ecdysone mediates the development of immunity in the Drosophila embryo
Abstract
Beyond their role in cell metabolism, development, and reproduction, hormones are also important modulators of the immune system. In the context of inflammatory disorders, systemic administration of pharmacological doses of synthetic glucocorticoids (GCs) is widely used as an anti-inflammatory treatment [1, 2]. However, not all actions of GCs are immunosuppressive, and many studies have suggested that physiological concentrations of GCs can have immunoenhancing effects [3-7]. For a more comprehensive understanding of how steroid hormones regulate immunity and inflammation, a simple in vivo system is required. The Drosophila embryo has recently emerged as a powerful model system to study the recruitment of immune cells to sterile wounds [8] and host-pathogen dynamics [9]. Here we investigate the immune response of the fly embryo to bacterial infections and find that the steroid hormone 20-hydroxyecdysone (20-HE) can regulate the quality of the immune response and influence the resolution of infection in Drosophila embryos.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
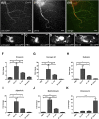
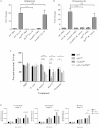
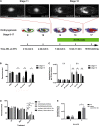
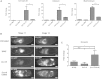
Similar articles
-
Ecdysone promotes gene- and pathogen-specific immune responses to Micrococcus luteus and Bacillus subtilis in Drosophila S2 cells.J Insect Physiol. 2024 Dec;159:104710. doi: 10.1016/j.jinsphys.2024.104710. Epub 2024 Sep 15. J Insect Physiol. 2024. PMID: 39288896
-
RNA interference directs innate immunity against viruses in adult Drosophila.Science. 2006 Apr 21;312(5772):452-4. doi: 10.1126/science.1125694. Epub 2006 Mar 23. Science. 2006. PMID: 16556799 Free PMC article.
-
Escherichia coli and Micrococcus luteus Activate the CG45045 Gene in Drosophila S2 Cell Line.Dokl Biochem Biophys. 2024 Dec;519(1):482-485. doi: 10.1134/S160767292460074X. Epub 2024 Oct 11. Dokl Biochem Biophys. 2024. PMID: 39400767
-
Ecdysone signaling cascade and regulation of Drosophila metamorphosis.Arch Insect Biochem Physiol. 1996;33(3-4):231-44. doi: 10.1002/(SICI)1520-6327(1996)33:3/4<231::AID-ARCH5>3.0.CO;2-V. Arch Insect Biochem Physiol. 1996. PMID: 8913033 Review.
-
Unraveling the Molecular Mechanism of Immunosenescence in Drosophila.Int J Mol Sci. 2018 Aug 21;19(9):2472. doi: 10.3390/ijms19092472. Int J Mol Sci. 2018. PMID: 30134574 Free PMC article. Review.
Cited by
-
As the fat flies: The dynamic lipid droplets of Drosophila embryos.Biochim Biophys Acta. 2015 Sep;1851(9):1156-85. doi: 10.1016/j.bbalip.2015.04.002. Epub 2015 Apr 13. Biochim Biophys Acta. 2015. PMID: 25882628 Free PMC article. Review.
-
The Anti-Inflammatory and Uric Acid Lowering Effects of Si-Miao-San on Gout.Front Immunol. 2022 Jan 5;12:777522. doi: 10.3389/fimmu.2021.777522. eCollection 2021. Front Immunol. 2022. PMID: 35069549 Free PMC article.
-
The extraembryonic serosa is a frontier epithelium providing the insect egg with a full-range innate immune response.Elife. 2014 Dec 9;3:e04111. doi: 10.7554/eLife.04111. Elife. 2014. PMID: 25487990 Free PMC article.
-
The Cat Flea (Ctenocephalides felis) Immune Deficiency Signaling Pathway Regulates Rickettsia typhi Infection.Infect Immun. 2017 Dec 19;86(1):e00562-17. doi: 10.1128/IAI.00562-17. Print 2018 Jan. Infect Immun. 2017. PMID: 29084898 Free PMC article.
-
Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiology.Nat Rev Immunol. 2014 Dec;14(12):796-810. doi: 10.1038/nri3763. Nat Rev Immunol. 2014. PMID: 25421701 Free PMC article. Review.
References
-
- Necela B.M., Cidlowski J.A. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc. Am. Thorac. Soc. 2004;1:239–246. - PubMed
-
- Galon J., Franchimont D., Hiroi N., Frey G., Boettner A., Ehrhart-Bornstein M., O’Shea J.J., Chrousos G.P., Bornstein S.R. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

