Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial
- PMID: 24794368
- PMCID: PMC4303045
- DOI: 10.1001/jama.2014.2623
Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial
Abstract
Importance: Biliary atresia is the most common cause of end-stage liver disease in children. Controversy exists as to whether use of steroids after hepatoportoenterostomy improves clinical outcome.
Objective: To determine whether the addition of high-dose corticosteroids after hepatoportoenterostomy is superior to surgery alone in improving biliary drainage and survival with the native liver.
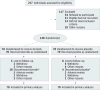
Design, setting, and patients: The multicenter, double-blind Steroids in Biliary Atresia Randomized Trial (START) was conducted in 140 infants (mean age, 2.3 months) between September 2005 and February 2011 in the United States; follow-up ended in January 2013.
Interventions: Participants were randomized to receive intravenous methylprednisolone (4 mg/kg/d for 2 weeks) and oral prednisolone (2 mg/kg/d for 2 weeks) followed by a tapering protocol for 9 weeks (n = 70) or placebo (n = 70) initiated within 72 hours of hepatoportoenterostomy.
Main outcomes and measures: The primary end point (powered to detect a 25% absolute treatment difference) was the percentage of participants with a serum total bilirubin level of less than 1.5 mg/dL with his/her native liver at 6 months posthepatoportoenterostomy. Secondary outcomes included survival with native liver at 24 months of age and serious adverse events.
Results: The proportion of participants with improved bile drainage was not statistically significantly improved by steroids at 6 months posthepatoportoenterostomy (58.6% [41/70] of steroids group vs 48.6% [34/70] of placebo group; adjusted relative risk, 1.14 [95% CI, 0.83 to 1.57]; P = .43). The adjusted absolute risk difference was 8.7% (95% CI, -10.4% to 27.7%). Transplant-free survival was 58.7% in the steroids group vs 59.4% in the placebo group (adjusted hazard ratio, 1.0 [95% CI, 0.6 to 1.8]; P = .99) at 24 months of age. The percentage of participants with serious adverse events was 81.4% [57/70] of the steroids group and 80.0% [56/70] of the placebo group (P > .99); however, participants receiving steroids had an earlier time of onset of their first serious adverse event by 30 days posthepatoportoenterostomy (37.2% [95% CI, 26.9% to 50.0%] of steroids group vs 19.0% [95% CI, 11.5% to 30.4%] of placebo group; P = .008).
Conclusions and relevance: Among infants with biliary atresia who have undergone hepatoportoenterostomy, high-dose steroid therapy following surgery did not result in statistically significant treatment differences in bile drainage at 6 months, although a small clinical benefit could not be excluded. Steroid treatment was associated with earlier onset of serious adverse events in children with biliary atresia.
Trial registration: clinicaltrials.gov Identifier: NCT00294684.
Conflict of interest statement
Figures
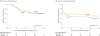
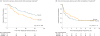
References
-
- Shneider BL, Brown MB, Haber B, et al. Biliary Atresia Research Consortium A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148(4):467–474. - PubMed
-
- Chardot C, Buet C, Serinet MO, et al. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. 2013;58(6):1209–1217. - PubMed
-
- Karrer FM, Lilly JR. Corticosteroid therapy in biliary atresia. J Pediatr Surg. 1985;20(6):693–695. - PubMed
-
- Kasai M, Suzuki H, Ohashi E, Ohi R, Chiba T, Okamoto A. Technique and results of operative management of biliary atresia. World J Surg. 1978;2(5):571–579. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 DK062481/DK/NIDDK NIH HHS/United States
- DK84536/DK/NIDDK NIH HHS/United States
- U01 DK062470/DK/NIDDK NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- DK84585/DK/NIDDK NIH HHS/United States
- U01 DK062466/DK/NIDDK NIH HHS/United States
- UL1 TR000424/TR/NCATS NIH HHS/United States
- U01 DK103149/DK/NIDDK NIH HHS/United States
- DK62436/DK/NIDDK NIH HHS/United States
- UL1 TR000130/TR/NCATS NIH HHS/United States
- DK62452/DK/NIDDK NIH HHS/United States
- TR000423/TR/NCATS NIH HHS/United States
- U01 DK084585/DK/NIDDK NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- TR000005/TR/NCATS NIH HHS/United States
- TR000130/TR/NCATS NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- U01 DK084538/DK/NIDDK NIH HHS/United States
- U01 DK062453/DK/NIDDK NIH HHS/United States
- U01 DK062503/DK/NIDDK NIH HHS/United States
- TR000003/TR/NCATS NIH HHS/United States
- DK62453/DK/NIDDK NIH HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- DK62481/DK/NIDDK NIH HHS/United States
- DK84575/DK/NIDDK NIH HHS/United States
- DK62470/DK/NIDDK NIH HHS/United States
- P30 DK078392/DK/NIDDK NIH HHS/United States
- U01 DK103135/DK/NIDDK NIH HHS/United States
- U01 DK062436/DK/NIDDK NIH HHS/United States
- TR000077/TR/NCATS NIH HHS/United States
- DK84538/DK/NIDDK NIH HHS/United States
- DK62466/DK/NIDDK NIH HHS/United States
- TR000007/TR/NCATS NIH HHS/United States
- U01 DK062452/DK/NIDDK NIH HHS/United States
- TR000004/TR/NCATS NIH HHS/United States
- DK62500/DK/NIDDK NIH HHS/United States
- TR000150/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- DK62497/DK/NIDDK NIH HHS/United States
- TR000154/TR/NCATS NIH HHS/United States
- U01 DK084575/DK/NIDDK NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- DK62456/DK/NIDDK NIH HHS/United States
- U01 DK084536/DK/NIDDK NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- TR000454/TR/NCATS NIH HHS/United States
- DK62445/DK/NIDDK NIH HHS/United States
- DK62503/DK/NIDDK NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- U01 DK062456/DK/NIDDK NIH HHS/United States
- TR000424/TR/NCATS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- U01 DK062445/DK/NIDDK NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- TR000448/TR/NCATS NIH HHS/United States
- U24 DK062456/DK/NIDDK NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 DK062500/DK/NIDDK NIH HHS/United States
- U01 DK062497/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

