High yield soluble bacterial expression and streamlined purification of recombinant human interferon α-2a
- PMID: 24794500
- PMCID: PMC4070333
- DOI: 10.1016/j.pep.2014.04.010
High yield soluble bacterial expression and streamlined purification of recombinant human interferon α-2a
Abstract
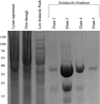
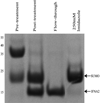
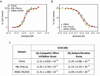
Interferon α-2a (IFNA2) is a member of the Type I interferon cytokine family, known for its antiviral and anti-proliferative functions. The role of this family in the innate immune response makes it an attractive candidate for the treatment of many viral and chronic immune-compromised diseases. Recombinant IFNA2 is clinically used to modulate hairy cell leukemia as well as hepatitis c. Historically, IFNA2 has been purified from human leukocytes as well as bacterial expression systems. In most cases, bacterial expression of IFNA2 resulted in inclusion body formation, or required numerous purification steps that decreased the protein yield. Here, we describe an expression and purification scheme for IFNA2 using a pET-SUMO bacterial expression system and a single purification step. Using the SUMO protein as the fusion tag achieved high soluble protein expression. The SUMO tag was cleaved with the Ulp1 protease leaving no additional amino acids on the fusion terminus following cleavage. Mass spectrometry, circular dichroism, 2D heteronuclear NMR, and analytical ultracentrifugation confirmed the amino acid sequence identity, secondary and tertiary protein structures, and the solution behavior of the purified IFNA2. The purified protein also had antiviral and anti-proliferative activities comparable to the WHO International Standard, NIBSC 95/650, and the IFNA2 standard available from PBL Assay Science. Combining the expression and purification protocols developed here to produce IFNA2 on a laboratory scale with the commercial fermenter technology commonly used in pharmaceutical industry may further enhance IFNA2 yields, which will promote the development of interferon-based protein drugs to treat various disorders.
Keywords: Bacterial expression; Interferon α-2a; Purification; SUMO; Soluble protein.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- Isaacs A, Lindenmann J. Virus Interference. I. The Interferon. Proceedings of the Royal Society of London. Series B - Biological Sciences. 1957;147(927):258–267. - PubMed
-
- Malmagaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439–454. - PubMed
-
- Fensterl V, Sen GC. Interferons and viral infections. Biofactors. 2009;35(1):14–20. - PubMed
-
- Kaser A, Tilg H. Interferon-α in inflammation and immunity. Cell Mol Bio. 2000;47:609–617. - PubMed
-
- Theofilopoulos AN, et al. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

