A two-tier Golgi-based control of organelle size underpins the functional plasticity of endothelial cells
- PMID: 24794632
- PMCID: PMC4022834
- DOI: 10.1016/j.devcel.2014.03.021
A two-tier Golgi-based control of organelle size underpins the functional plasticity of endothelial cells
Abstract
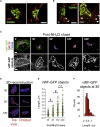
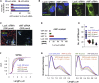
Weibel-Palade bodies (WPBs), endothelial-specific secretory granules that are central to primary hemostasis and inflammation, occur in dimensions ranging between 0.5 and 5 μm. How their size is determined and whether it has a functional relevance are at present unknown. Here, we provide evidence for a dual role of the Golgi apparatus in controlling the size of these secretory carriers. At the ministack level, cisternae constrain the size of nanostructures ("quanta") of von Willebrand factor (vWF), the main WPB cargo. The ribbon architecture of the Golgi then allows copackaging of a variable number of vWF quanta within the continuous lumen of the trans-Golgi network, thereby generating organelles of different sizes. Reducing the WPB size abates endothelial cell hemostatic function by drastically diminishing platelet recruitment, but, strikingly, the inflammatory response (the endothelial capacity to engage leukocytes) is unaltered. Size can thus confer functional plasticity to an organelle by differentially affecting its activities.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Comment in
-
The Golgi is a measuring cup.Dev Cell. 2014 May 12;29(3):259-60. doi: 10.1016/j.devcel.2014.04.028. Dev Cell. 2014. PMID: 24823373 Free PMC article.
References
-
- Bonfanti R., Furie B.C., Furie B., Wagner D.D. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. - PubMed
-
- Dacks J.B., Field M.C. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J. Cell Sci. 2007;120:2977–2985. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

