Role of autophagy in cisplatin resistance in ovarian cancer cells
- PMID: 24794870
- PMCID: PMC4059157
- DOI: 10.1074/jbc.M114.558288
Role of autophagy in cisplatin resistance in ovarian cancer cells
Abstract
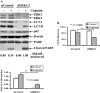
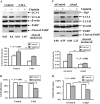
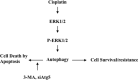
Cisplatin-based treatment is the first line chemotherapy for several cancers including ovarian cancer. The development of cisplatin resistance results in treatment failure, but the underlying mechanisms are not fully understood. Here we show that the induction of autophagy plays an important role in cisplatin resistance in ovarian cancer cells. Specifically, we show that cisplatin resistance is correlated with autophagy induction in a panel of ovarian cancer cells but not in immortalized human ovarian surface epithelial cells. Mechanistically, cisplatin treatment activates ERK and subsequently promotes autophagy. The inhibition of ERK activation with MEK inhibitors or knockdown of ERK expression with siRNA decreases cisplatin-induced autophagy and subsequently sensitizes ovarian cancer cells to cisplatin-induced apoptosis. In ovarian cancer cells that have developed acquired cisplatin resistance, both ERK activation and autophagy induction are increased. Importantly, knockdown of ERK or inhibition of autophagy promotes cisplatin-induced apoptosis in acquired cisplatin-resistant cells. Collectively, our data indicate that ERK-mediated autophagy can lead to cisplatin resistance and suggest that cisplatin resistance can be overcome by inhibition of autophagy in ovarian cancer cells.
Keywords: Autophagy; Cancer Therapy; Drug Resistance; Extracellular Signal-regulated Kinase (ERK); Ovarian Cancer.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Bryant C. S., Kumar S., Spannuth W., Shah J. P., Munkarah A. R., Deppe G., Alvarez R. D., Morris R. T. (2011) Feasibility of extension of platinum-free interval with weekly bolus topotecan and subsequent platinum retreatment outcomes in recurrent ovarian cancer. Arch. Gyn. Obstet. 283, 361–367 - PubMed
-
- Tummala M. K., McGuire W. P. (2005) Recurrent ovarian cancer. Clin. Adv. Hematol. Oncol. 3, 723–736 - PubMed
-
- Go R. S., Adjei A. A. (1999) Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J. Clin. Oncol. 17, 409–422 - PubMed
-
- Kelland L. (2007) The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7, 573–584 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

