Contribution of pyruvate phosphate dikinase in the maintenance of the glycosomal ATP/ADP balance in the Trypanosoma brucei procyclic form
- PMID: 24794874
- PMCID: PMC4067170
- DOI: 10.1074/jbc.M114.567230
Contribution of pyruvate phosphate dikinase in the maintenance of the glycosomal ATP/ADP balance in the Trypanosoma brucei procyclic form
Abstract
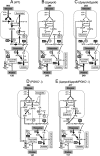
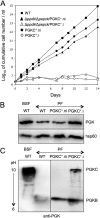
Trypanosoma brucei belongs to a group of protists that sequester the first six or seven glycolytic steps inside specialized peroxisomes, named glycosomes. Because of the glycosomal membrane impermeability to nucleotides, ATP molecules consumed by the first glycolytic steps need to be regenerated in the glycosomes by kinases, such as phosphoenolpyruvate carboxykinase (PEPCK). The glycosomal pyruvate phosphate dikinase (PPDK), which reversibly converts phosphoenolpyruvate into pyruvate, could also be involved in this process. To address this question, we analyzed the metabolism of the main carbon sources used by the procyclic trypanosomes (glucose, proline, and threonine) after deletion of the PPDK gene in the wild-type (Δppdk) and PEPCK null (Δppdk/Δpepck) backgrounds. The rate of acetate production from glucose is 30% reduced in the Δppdk mutant, whereas threonine-derived acetate production is not affected, showing that PPDK function in the glycolytic direction with production of ATP in the glycosomes. The Δppdk/Δpepck mutant incubated in glucose as the only carbon source showed a 3.8-fold reduction of the glycolytic rate compared with the Δpepck mutant, as a consequence of the imbalanced glycosomal ATP/ADP ratio. The role of PPDK in maintenance of the ATP/ADP balance was confirmed by expressing the glycosomal phosphoglycerate kinase (PGKC) in the Δppdk/Δpepck cell line, which restored the glycolytic flux. We also observed that expression of PGKC is lethal for procyclic trypanosomes, as a consequence of ATP depletion, due to glycosomal relocation of cytosolic ATP production. This illustrates the key roles played by glycosomal and cytosolic kinases, including PPDK, to maintain the cellular ATP/ADP homeostasis.
Keywords: Gene Knockout; Glucose Metabolism; Parasite Metabolism; Peroxisome; Trypanosoma brucei.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Marshall J. S., Ashton A. R., Govers F., Hardham A. R. (2001) Isolation and characterization of four genes encoding pyruvate, phosphate dikinase in the oomycete plant pathogen Phytophthora cinnamomi. Curr. Genet. 40, 73–81 - PubMed
-
- Nevalainen L., Hrdý I., Müller M. (1996) Sequence of a Giardia lamblia gene coding for the glycolytic enzyme, pyruvate, phosphate dikinase. Mol. Biochem. Parasitol. 77, 217–223 - PubMed
-
- Feng X. M., Cao L. J., Adam R. D., Zhang X. C., Lu S. Q. (2008) The catalyzing role of PPDK in Giardia lamblia. Biochem. Biophys. Res. Commun. 367, 394–398 - PubMed
-
- Saavedra E., Encalada R., Pineda E., Jasso-Chávez R., Moreno-Sánchez R. (2005) Glycolysis in Entamoeba histolytica. Biochemical characterization of recombinant glycolytic enzymes and flux control analysis. FEBS J. 272, 1767–1783 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

