Antimicrobial prophylaxis for children with vesicoureteral reflux
- PMID: 24795142
- PMCID: PMC4137319
- DOI: 10.1056/NEJMoa1401811
Antimicrobial prophylaxis for children with vesicoureteral reflux
Abstract
Background: Children with febrile urinary tract infection commonly have vesicoureteral reflux. Because trial results have been limited and inconsistent, the use of antimicrobial prophylaxis to prevent recurrences in children with reflux remains controversial.
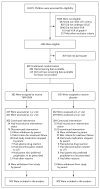
Methods: In this 2-year, multisite, randomized, placebo-controlled trial involving 607 children with vesicoureteral reflux that was diagnosed after a first or second febrile or symptomatic urinary tract infection, we evaluated the efficacy of trimethoprim-sulfamethoxazole prophylaxis in preventing recurrences (primary outcome). Secondary outcomes were renal scarring, treatment failure (a composite of recurrences and scarring), and antimicrobial resistance.
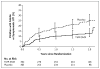
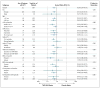
Results: Recurrent urinary tract infection developed in 39 of 302 children who received prophylaxis as compared with 72 of 305 children who received placebo (relative risk, 0.55; 95% confidence interval [CI], 0.38 to 0.78). Prophylaxis reduced the risk of recurrences by 50% (hazard ratio, 0.50; 95% CI, 0.34 to 0.74) and was particularly effective in children whose index infection was febrile (hazard ratio, 0.41; 95% CI, 0.26 to 0.64) and in those with baseline bladder and bowel dysfunction (hazard ratio, 0.21; 95% CI, 0.08 to 0.58). The occurrence of renal scarring did not differ significantly between the prophylaxis and placebo groups (11.9% and 10.2%, respectively). Among 87 children with a first recurrence caused by Escherichia coli, the proportion of isolates that were resistant to trimethoprim-sulfamethoxazole was 63% in the prophylaxis group and 19% in the placebo group.
Conclusions: Among children with vesicoureteral reflux after urinary tract infection, antimicrobial prophylaxis was associated with a substantially reduced risk of recurrence but not of renal scarring. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; RIVUR ClinicalTrials.gov number, NCT00405704.).
Figures
Comment in
-
Antibiotic prophylaxis for vesicoureteral reflux--answers, yet questions.N Engl J Med. 2014 Jun 19;370(25):2440-1. doi: 10.1056/NEJMe1404774. Epub 2014 May 4. N Engl J Med. 2014. PMID: 24795143 No abstract available.
-
Paediatrics: antimicrobial prophylaxis for vesicoureteral reflux-where will the RIVUR study lead us?Nat Rev Urol. 2014 Jun;11(6):301. doi: 10.1038/nrurol.2014.112. Epub 2014 May 20. Nat Rev Urol. 2014. PMID: 24841170 No abstract available.
-
RIVUR trial offers confirmatory evidence for a small but real benefit of antibiotics for UTI prevention in children.Evid Based Med. 2014 Dec;19(6):229-30. doi: 10.1136/ebmed-2014-110070. Epub 2014 Sep 4. Evid Based Med. 2014. PMID: 25189165 No abstract available.
-
Antimicrobial prophylaxis for children with vesicoureteral reflux.N Engl J Med. 2014 Sep 11;371(11):1072-3. doi: 10.1056/NEJMc1408559. N Engl J Med. 2014. PMID: 25207771 No abstract available.
-
Antimicrobial prophylaxis for children with vesicoureteral reflux.N Engl J Med. 2014 Sep 11;371(11):1070. doi: 10.1056/NEJMc1408559. N Engl J Med. 2014. PMID: 25207772 No abstract available.
-
Antimicrobial prophylaxis for children with vesicoureteral reflux.N Engl J Med. 2014 Sep 11;371(11):1071. doi: 10.1056/NEJMc1408559. N Engl J Med. 2014. PMID: 25207773 No abstract available.
-
Antimicrobial prophylaxis for children with vesicoureteral reflux.N Engl J Med. 2014 Sep 11;371(11):1071. doi: 10.1056/NEJMc1408559. N Engl J Med. 2014. PMID: 25207774 No abstract available.
-
Antimicrobial prophylaxis for children with vesicoureteral reflux.N Engl J Med. 2014 Sep 11;371(11):1071-2. doi: 10.1056/NEJMc1408559. N Engl J Med. 2014. PMID: 25207775 No abstract available.
-
Vesikoureteraler Reflux - Profitieren Kinder von einer Antibiotikaprophylaxe?Aktuelle Urol. 2014 Sep;45(5):337-40. doi: 10.1055/s-0034-1393910. Epub 2014 Oct 2. Aktuelle Urol. 2014. PMID: 25275679 German. No abstract available.
-
Narrowing the focus: what we now know (and still don't know) about antibiotic prophylaxis for children with vesicoureteral reflux.Am J Kidney Dis. 2015 Feb;65(2):214-6. doi: 10.1053/j.ajkd.2014.09.004. Epub 2014 Oct 29. Am J Kidney Dis. 2015. PMID: 25441431 No abstract available.
-
Antibiotic prophylaxis prevents urinary tract infection recurrence.J Pediatr. 2015 Mar;166(3):778. doi: 10.1016/j.jpeds.2014.12.046. J Pediatr. 2015. PMID: 25722276 No abstract available.
-
Antibiotic prophylaxis in children with vesicoureteric reflux: has RIVUR answered all our questions?Ir Med J. 2015 Apr;108(4):101-2. Ir Med J. 2015. PMID: 26016296 No abstract available.
References
-
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195–202. - PubMed
-
- Medical versus surgical treatment of primary vesicoureteral reflux: report of the International Reflux Study Committee. Pediatrics. 1981;67:392–400. - PubMed
-
- Smellie JM, Barratt TM, Chantler C, et al. Medical versus surgical treatment in children with severe bilateral vesicoureteric reflux and bilateral nephropathy: a randomised trial. Lancet. 2001;357:1329–33. - PubMed
-
- Weiss R, Duckett J, Spitzer A. Results of a randomized clinical trial of medical versus surgical management of infants and children with grades III and IV primary vesicoureteral reflux (United States) J Urol. 1992;148:1667–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 DK074062/DK/NIDDK NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- U01 DK074082/DK/NIDDK NIH HHS/United States
- U01 DK074063/DK/NIDDK NIH HHS/United States
- UL1TR000005/TR/NCATS NIH HHS/United States
- U01 DK074059/DK/NIDDK NIH HHS/United States
- U01 DK074053/DK/NIDDK NIH HHS/United States
- U01 DK074064/DK/NIDDK NIH HHS/United States
- UL1TR000003/TR/NCATS NIH HHS/United States
- UL1RR024153/RR/NCRR NIH HHS/United States
- K23 DK088943/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
