Functional anatomy and ion regulatory mechanisms of the antennal gland in a semi-terrestrial crab, Ocypode stimpsoni
- PMID: 24795144
- PMCID: PMC4058075
- DOI: 10.1242/bio.20147336
Functional anatomy and ion regulatory mechanisms of the antennal gland in a semi-terrestrial crab, Ocypode stimpsoni
Abstract
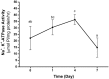
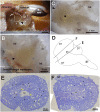
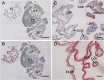
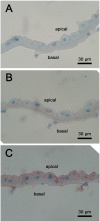
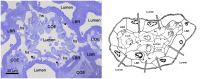
Brachyuran crabs from diverse habitats show great differences in their osmoregulatory processes, especially in terms of the structural and physiological characteristics of the osmoregulatory organs. In crustaceans, the antennal glands are known to be important in osmoregulation, and they play a functional role analogous to that of the vertebrate kidney. Nevertheless, the detailed structure and function of the antennal glands in different species have rarely been described. The aim of this study is to investigate the role of the antennal gland in ion regulation by examining the ultrastructure of the cells and the distribution of the ion regulatory proteins in each cell type in the antennal gland of a semi-terrestrial crab. The results showed that Na(+), K(+)-ATPase activity significantly increased in the antennal gland after a 4-day acclimation in dilute seawater and returned to its original (day 0) level after 7 days. Three major types of cells were identified in the antennal gland, including coelomic cells (COEs), labyrinthine cells (LBRs) and end-labyrinthine cells (ELBRs). The proximal tubular region (PT) and distal tubular region (DT) of the antennal gland consist of LBRs and COEs, whereas the end tubular region (ET) consists of all three types of cells, with fewer COEs and more ELBRs. We found a non-uniform distribution of NKA immunoreactivity, with increasing intensity from the proximal to the distal regions of the antennal gland. We summarise our study with a proposed model for the urine reprocessing pathway and the role of each cell type or segment of the antennal gland.
Keywords: Antennal gland; Coelomic cell; K+-ATPase; Labyrinthine cell; Na+; Osmoregulation.
© 2014. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures




Similar articles
-
A Multi-Species Comparison and Evolutionary Perspectives on Ion Regulation in the Antennal Gland of Brachyurans.Front Physiol. 2022 Jun 2;13:902937. doi: 10.3389/fphys.2022.902937. eCollection 2022. Front Physiol. 2022. PMID: 35721559 Free PMC article. Review.
-
Analysis of adaptive molecular mechanisms in response to low salinity in antennal gland of mud crab, Scylla paramamosain.Heliyon. 2024 Feb 5;10(3):e25556. doi: 10.1016/j.heliyon.2024.e25556. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38356600 Free PMC article.
-
Ion regulation in the antennal glands differs among Ocypodoidea and Grapsoidea crab species.Comp Biochem Physiol A Mol Integr Physiol. 2020 Oct;248:110753. doi: 10.1016/j.cbpa.2020.110753. Epub 2020 Jul 10. Comp Biochem Physiol A Mol Integr Physiol. 2020. PMID: 32653510
-
V-type H+-ATPase and Na+,K+-ATPase in the gills of 13 euryhaline crabs during salinity acclimation.J Exp Biol. 2007 Feb;210(Pt 4):620-7. doi: 10.1242/jeb.02684. J Exp Biol. 2007. PMID: 17267648
-
Ionic regulatory strategies of crabs: the transition from water to land.Front Physiol. 2024 Sep 27;15:1399194. doi: 10.3389/fphys.2024.1399194. eCollection 2024. Front Physiol. 2024. PMID: 39397859 Free PMC article. Review.
Cited by
-
Histopathological and Proteomics Analysis of Shrimp Litopenaeus vannamei Infected with Ecytonucleospora hepatopenaei.Microorganisms. 2025 Feb 12;13(2):402. doi: 10.3390/microorganisms13020402. Microorganisms. 2025. PMID: 40005768 Free PMC article.
-
A Multi-Species Comparison and Evolutionary Perspectives on Ion Regulation in the Antennal Gland of Brachyurans.Front Physiol. 2022 Jun 2;13:902937. doi: 10.3389/fphys.2022.902937. eCollection 2022. Front Physiol. 2022. PMID: 35721559 Free PMC article. Review.
-
Anatomical and molecular insights into the antennal gland of the giant freshwater prawn Macrobrachium rosenbergii.Cell Tissue Res. 2024 Aug;397(2):125-146. doi: 10.1007/s00441-024-03898-3. Epub 2024 Jun 15. Cell Tissue Res. 2024. PMID: 38878176 Free PMC article.
-
Analysis of adaptive molecular mechanisms in response to low salinity in antennal gland of mud crab, Scylla paramamosain.Heliyon. 2024 Feb 5;10(3):e25556. doi: 10.1016/j.heliyon.2024.e25556. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38356600 Free PMC article.
-
Epizootic shell disease induces systemic transcriptomic shifts in Homarus americanus, characterized by increased shell degradation and impaired energy metabolism across tissues.Front Physiol. 2025 Aug 1;16:1642696. doi: 10.3389/fphys.2025.1642696. eCollection 2025. Front Physiol. 2025. PMID: 40821945 Free PMC article.
References
-
- Ahearn G. A., Franco P. (1990). Sodium and calcium share the electrogenic 2 Na(+)-1 H+ antiporter in crustacean antennal glands. Am. J. Physiol. 259, F758–F767. - PubMed
-
- Buranajitpirom D., Asuvapongpatana S., Weerachatyanukul W., Wongprasert K., Namwong W., Poltana P., Withyachumnarnkul B. (2010). Adaptation of the black tiger shrimp, Penaeus monodon, to different salinities through an excretory function of the antennal gland. Cell Tissue Res. 340, 481–489 10.1007/s00441-010-0971-y - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

