EphA receptors regulate prostate cancer cell dissemination through Vav2-RhoA mediated cell-cell repulsion
- PMID: 24795148
- PMCID: PMC4058079
- DOI: 10.1242/bio.20146601
EphA receptors regulate prostate cancer cell dissemination through Vav2-RhoA mediated cell-cell repulsion
Abstract
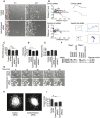
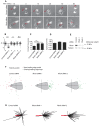
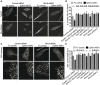
Metastatic prostate cancer cells display EphB receptor-mediated attraction when they contact stromal fibroblasts but EphA-driven repulsion when they contact one another. The impact of these 'social' interactions between cells during cancer cell invasion and the signalling mechanisms downstream of Eph receptors are unclear. Here we show that EphA receptors regulate prostate cancer cell dissemination in a 2D dispersal assay and in a 3D cancer cell spheroid assay. We show that EphA receptors signal via the exchange factor Vav2 to activate RhoA and that both Vav2 and RhoA are required for prostate cancer cell-cell repulsion. Furthermore, we find that in EphA2/EphA4, Vav2 or RhoA siRNA-treated cells, contact repulsion can be restored by partial microtubule destabilisation. We propose that EphA-Vav2-RhoA-mediated repulsion between contacting cancer cells at the tumour edge could enhance their local invasion away from the primary tumour.
Keywords: Cell migration; Contact inhibition of locomotion; Eph receptors; Prostate cancer; Rho GTPases; Vav2.
© 2014. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

