Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells
- PMID: 24795456
- PMCID: PMC4025611
- DOI: 10.4049/jimmunol.1302492
Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells
Abstract
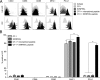
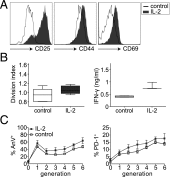
Until recently, the known roles of lymphatic endothelial cells (LECs) in immune modulation were limited to directing immune cell trafficking and passively transporting peripheral Ags to lymph nodes. Recent studies demonstrated that LECs can directly suppress dendritic cell maturation and present peripheral tissue and tumor Ags for autoreactive T cell deletion. We asked whether LECs play a constitutive role in T cell deletion under homeostatic conditions. In this study, we demonstrate that murine LECs under noninflamed conditions actively scavenge and cross-present foreign exogenous Ags to cognate CD8(+) T cells. This cross-presentation was sensitive to inhibitors of lysosomal acidification and endoplasmic reticulum-golgi transport and was TAP1 dependent. Furthermore, LECs upregulated MHC class I and the PD-1 ligand PD-L1, but not the costimulatory molecules CD40, CD80, or CD86, upon Ag-specific interactions with CD8(+) T cells. Finally, Ag-specific CD8(+) T cells that were activated by LECs underwent proliferation, with early-generation apoptosis and dysfunctionally activated phenotypes that could not be reversed by exogenous IL-2. These findings help to establish LECs as APCs that are capable of scavenging and cross-presenting exogenous Ags, in turn causing dysfunctional activation of CD8(+) T cells under homeostatic conditions. Thus, we suggest that steady-state lymphatic drainage may contribute to peripheral tolerance by delivering self-Ags to lymph node-resident leukocytes, as well as by providing constant exposure of draining peripheral Ags to LECs, which maintain tolerogenic cross-presentation of such Ags.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

