Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment
- PMID: 24795473
- PMCID: PMC4192039
- DOI: 10.1093/infdis/jiu254
Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment
Abstract
Background: Defining the association of non-AIDS-defining events with inflammation and immune activation among human immunodeficiency virus (HIV)-infected persons with antiretroviral therapy (ART)-associated virological suppression is critical to identifying interventions to decrease the occurrence of these events.
Methods: We conducted a case-control study of HIV-infected subjects who had achieved virological suppression within 1 year after ART initiation. Cases were patients who experienced non-AIDS-defining events, defined as myocardial infarction, stroke, non-AIDS-defining cancer, non-AIDS-defining serious bacterial infection, or death. Controls were matched to cases on the basis of age, sex, pre-ART CD4(+) T-cell count, and ART regimen. Peripheral blood mononuclear cells and plasma specimens obtained at the visit before ART initiation (hereafter, baseline), the visit approximately 1 year after ART initiation (hereafter, year 1), and the visit immediately preceding the non-AIDS-defining event (hereafter, pre-event) were analyzed for activated CD4(+) and CD8(+) T cells, plasma interleukin 6 (IL-6) level, soluble tumor necrosis factor receptor I (sTNFR-I) level, sTNFR-II level, soluble CD14 level, kynurenine-to-tryptophan (KT) ratio, and D-dimer level. Conditional logistic regression analysis was used to study the association between biomarkers and outcomes, with adjustment for potential confounders.
Results: Higher IL-6 level, sTNFR-I level, sTNFR-II level, KT ratio, and D-dimer level at year 1 were associated with the occurrence of a non-AIDS-defining event. Significant associations were also seen between non-AIDS-defining events and values of these biomarkers in specimens obtained at baseline and the pre-event time points. Effects remained significant after control for confounders. T-cell activation was not significantly related to outcomes.
Conclusions: Interventional trials to decrease the incidence of non-AIDS-defining events among HIV-infected persons with virological suppression should consider targeting the pathways represented by these soluble markers. Clinical Trials Registration. NCT00001137.
Keywords: ART; HIV; Non-AIDS morbidity; immune activation; inflammation.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
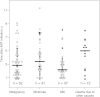
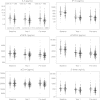


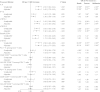

Comment in
-
Immunophenotype and function of CD38-expressing CD4+ and CD8+ T cells in HIV-infected patients undergoing suppressive combination antiretroviral therapy.J Infect Dis. 2015 May 1;211(9):1511-3. doi: 10.1093/infdis/jiu634. Epub 2014 Nov 14. J Infect Dis. 2015. PMID: 25398457 No abstract available.
-
Reply to Cannizzo et al.J Infect Dis. 2015 May 1;211(9):1514-5. doi: 10.1093/infdis/jiu635. Epub 2014 Nov 14. J Infect Dis. 2015. PMID: 25398458 No abstract available.
References
-
- Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–36. - PubMed
-
- Klein D, Hurley LB, Quesenberry CP, Jr., Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30:471–7. - PubMed
-
- Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- P30 MH062246/MH/NIMH NIH HHS/United States
- AI36219/AI/NIAID NIH HHS/United States
- K24 AI069994/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- R21 AI087035/AI/NIAID NIH HHS/United States
- AI068636/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

