The pregnane xenobiotic receptor, a prominent liver factor, has actions in the midbrain for neurosteroid synthesis and behavioral/neural plasticity of female rats
- PMID: 24795576
- PMCID: PMC4001026
- DOI: 10.3389/fnsys.2014.00060
The pregnane xenobiotic receptor, a prominent liver factor, has actions in the midbrain for neurosteroid synthesis and behavioral/neural plasticity of female rats
Abstract
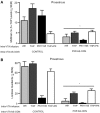
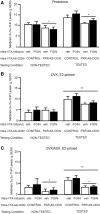
A novel factor of interest for growth/plasticity in the brain is pregnane xenobiotic receptor (PXR). PXR is a liver factor known for its role in xenobiotic clearance and cholesterol metabolism. It is expressed in the brain, suggesting a potential role for plasticity, particularly involving cholesterol-based steroids and neurosteroids. Mating induces synthesis of neurosteroids in the midbrain Ventral Tegmental Area (VTA) of female rodents, as well as other "plastic" regions of the brain, including the hippocampus, that may be involved in the consolidation of the mating experience. Reducing PXR in the VTA attenuates mating-induced biosynthesis of the neurosteroid, 5α-pregnan-3α-ol-20-one (3α,5α-THP). The 18 kDA translocator protein (TSPO) is one rate-limiting factor for 3α,5α-THP neurosteroidogenesis. The hypothesis tested was that PXR is an upstream factor of TSPO for neurosteroidogenesis of 3α,5α-THP in the VTA for lordosis, independent of peripheral glands. First, proestrous rats were administered a TSPO blocker (PK11195) and/or 3α,5α-THP following infusions of PXR antisense oligonucleotides (AS-ODNs) or vehicle to the VTA. Inhibiting TSPO with PK11195 reduced 3α,5α-THP levels in the midbrain and lordosis, an effect that could be reversed with 3α,5α-THP administration, but not AS-ODN+3α,5α-THP. Second, proestrous, ovariectomized (OVX), or ovariectomized/adrenalectomized (OVX/ADX) rats were infused with a TSPO enhancer (FGIN 1-27) subsequent to AS-ODNs or vehicle to the VTA. PXR AS-ODNs blocked actions of FGIN 1-27 for lordosis and 3α,5α-THP levels among proestrous > OVX > OVX/ADX rats. Thus, PXR may be upstream of TSPO, involved in neurosteroidogenesis of 3α,5α-THP in the brain for plasticity. This novel finding of a liver factor involved in behavioral/neural plasticity substantiates future studies investigating factors known for their prominent actions in the peripheral organs, such as the liver, for modulating brain function and its augmentation.
Keywords: allopregnanolone; cognition; midbrain ventral tegmental area; progesterone; reproduction.
Figures


Similar articles
-
Motivated behaviors and levels of 3α,5α-THP in the midbrain are attenuated by knocking down expression of pregnane xenobiotic receptor in the midbrain ventral tegmental area of proestrous rats.J Sex Med. 2013 Jul;10(7):1692-706. doi: 10.1111/jsm.12173. Epub 2013 May 1. J Sex Med. 2013. PMID: 23634744 Free PMC article.
-
Involvement of pregnane xenobiotic receptor in mating-induced allopregnanolone formation in the midbrain and hippocampus and brain-derived neurotrophic factor in the hippocampus among female rats.Psychopharmacology (Berl). 2014 Sep;231(17):3375-90. doi: 10.1007/s00213-014-3569-3. Epub 2014 May 1. Psychopharmacology (Berl). 2014. PMID: 24781516 Free PMC article.
-
Role of pregnane xenobiotic receptor in the midbrain ventral tegmental area for estradiol- and 3α,5α-THP-facilitated lordosis of female rats.Psychopharmacology (Berl). 2014 Sep;231(17):3365-74. doi: 10.1007/s00213-013-3406-0. Epub 2014 Jan 17. Psychopharmacology (Berl). 2014. PMID: 24435323 Free PMC article.
-
Pregnane xenobiotic receptors and membrane progestin receptors: role in neurosteroid-mediated motivated behaviours.J Neuroendocrinol. 2013 Nov;25(11):1002-11. doi: 10.1111/jne.12105. J Neuroendocrinol. 2013. PMID: 24028379 Free PMC article. Review.
-
Novel substrates for, and sources of, progestogens for reproduction.J Neuroendocrinol. 2011 Nov;23(11):961-73. doi: 10.1111/j.1365-2826.2011.02180.x. J Neuroendocrinol. 2011. PMID: 21696472 Free PMC article. Review.
Cited by
-
Overview of the Molecular Steps in Steroidogenesis of the GABAergic Neurosteroids Allopregnanolone and Pregnanolone.Chronic Stress (Thousand Oaks). 2018 Dec 19;2:2470547018818555. doi: 10.1177/2470547018818555. eCollection 2018 Jan-Dec. Chronic Stress (Thousand Oaks). 2018. PMID: 32440589 Free PMC article. Review.
-
Progesterone and its metabolite allopregnanolone promote invasion of human glioblastoma cells through metalloproteinase‑9 and cSrc kinase.Oncol Lett. 2023 Apr 13;25(6):223. doi: 10.3892/ol.2023.13809. eCollection 2023 Jun. Oncol Lett. 2023. PMID: 37153033 Free PMC article.
-
Pregnane X Receptor Deletion Modifies Recognition Memory and Electroencephalographic Activity.Neuroscience. 2018 Feb 1;370:130-138. doi: 10.1016/j.neuroscience.2017.07.038. Epub 2017 Jul 23. Neuroscience. 2018. PMID: 28743453 Free PMC article.
-
Allopregnanolone: Metabolism, Mechanisms of Action, and Its Role in Cancer.Int J Mol Sci. 2022 Dec 29;24(1):560. doi: 10.3390/ijms24010560. Int J Mol Sci. 2022. PMID: 36614002 Free PMC article. Review.
-
Additional pathways of sterol metabolism: Evidence from analysis of Cyp27a1-/- mouse brain and plasma.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Feb;1864(2):191-211. doi: 10.1016/j.bbalip.2018.11.006. Epub 2018 Nov 22. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30471425 Free PMC article.
References
-
- Acosta S. A., Tajiri N., Shinozuka K., Ishikawa H., Grimmig B., Diamond D., et al. (2013). Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS ONE 8:e53376 10.1371/journal.pone.0053376 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

