Sleep and olfactory cortical plasticity
- PMID: 24795585
- PMCID: PMC4001050
- DOI: 10.3389/fnbeh.2014.00134
Sleep and olfactory cortical plasticity
Abstract
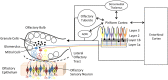
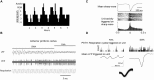
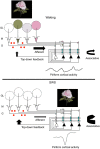
In many systems, sleep plays a vital role in memory consolidation and synaptic homeostasis. These processes together help store information of biological significance and reset synaptic circuits to facilitate acquisition of information in the future. In this review, we describe recent evidence of sleep-dependent changes in olfactory system structure and function which contribute to odor memory and perception. During slow-wave sleep, the piriform cortex becomes hypo-responsive to odor stimulation and instead displays sharp-wave activity similar to that observed within the hippocampal formation. Furthermore, the functional connectivity between the piriform cortex and other cortical and limbic regions is enhanced during slow-wave sleep compared to waking. This combination of conditions may allow odor memory consolidation to occur during a state of reduced external interference and facilitate association of odor memories with stored hedonic and contextual cues. Evidence consistent with sleep-dependent odor replay within olfactory cortical circuits is presented. These data suggest that both the strength and precision of odor memories is sleep-dependent. The work further emphasizes the critical role of synaptic plasticity and memory in not only odor memory but also basic odor perception. The work also suggests a possible link between sleep disturbances that are frequently co-morbid with a wide range of pathologies including Alzheimer's disease, schizophrenia and depression and the known olfactory impairments associated with those disorders.
Keywords: memory consolidation; odor memory; odor perception; olfaction; piriform cortex; slow-wave sleep.
Figures
Similar articles
-
Slow-wave sleep-imposed replay modulates both strength and precision of memory.J Neurosci. 2014 Apr 9;34(15):5134-42. doi: 10.1523/JNEUROSCI.5274-13.2014. J Neurosci. 2014. PMID: 24719093 Free PMC article.
-
Odor fear conditioning modifies piriform cortex local field potentials both during conditioning and during post-conditioning sleep.PLoS One. 2011 Mar 23;6(3):e18130. doi: 10.1371/journal.pone.0018130. PLoS One. 2011. PMID: 21448432 Free PMC article.
-
Sharp wave-associated activity patterns of cortical neurons in the mouse piriform cortex.Eur J Neurosci. 2018 Nov;48(10):3246-3254. doi: 10.1111/ejn.14099. Epub 2018 Oct 10. Eur J Neurosci. 2018. PMID: 30075483
-
Olfactory insights into sleep-dependent learning and memory.Prog Brain Res. 2014;208:309-43. doi: 10.1016/B978-0-444-63350-7.00012-7. Prog Brain Res. 2014. PMID: 24767488 Review.
-
The role of sleep in the plasticity of the olfactory system.Neurosci Res. 2017 May;118:21-29. doi: 10.1016/j.neures.2017.03.014. Epub 2017 May 10. Neurosci Res. 2017. PMID: 28501498 Review.
Cited by
-
Reciprocal relationships between sleep and smell.Front Neural Circuits. 2022 Dec 22;16:1076354. doi: 10.3389/fncir.2022.1076354. eCollection 2022. Front Neural Circuits. 2022. PMID: 36619661 Free PMC article. Review.
-
On the state-dependent nature of odor perception.Front Neurosci. 2022 Aug 26;16:964742. doi: 10.3389/fnins.2022.964742. eCollection 2022. Front Neurosci. 2022. PMID: 36090268 Free PMC article. Review.
-
Differences in the consolidation by spontaneous and evoked ripples in the presence of active dendrites.PLoS Comput Biol. 2024 Jun 25;20(6):e1012218. doi: 10.1371/journal.pcbi.1012218. eCollection 2024 Jun. PLoS Comput Biol. 2024. PMID: 38917228 Free PMC article.
-
Nitric Oxide-Mediated Modulation of Central Network Dynamics during Olfactory Perception.PLoS One. 2015 Sep 11;10(9):e0136846. doi: 10.1371/journal.pone.0136846. eCollection 2015. PLoS One. 2015. PMID: 26360020 Free PMC article.
-
The relationships between the clinical and polysomnographic findings and the olfactory function in patients with obstructive sleep apnea syndrome.Sleep Breath. 2015 Dec;19(4):1301-7. doi: 10.1007/s11325-015-1165-3. Epub 2015 Apr 9. Sleep Breath. 2015. PMID: 25855470
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

