Serine racemase: a key player in apoptosis and necrosis
- PMID: 24795622
- PMCID: PMC4000995
- DOI: 10.3389/fnsyn.2014.00009
Serine racemase: a key player in apoptosis and necrosis
Abstract
A fine balance between cell survival and cell death is required to sculpt the nervous system during development. However, an excess of cell death can occur following trauma, exposure to neurotoxins or alcohol, and some developmental and neurodegenerative diseases, such as Alzheimer's disease (AD). N-Methyl-D-aspartate receptors (NMDARs) support synaptic plasticity and survival of many neuronal populations whereas inappropriate activation may promote various forms of cell death, apoptosis, and necrosis representing the two extremes of a continuum of cell death processes both "in vitro" and "in vivo." Hence, by identifying the switches controlling pro-survival vs. apoptosis and apoptosis vs. pro-excitotoxic outcome of NMDAR stimulation, NMDAR modulators could be developed that selectively block the cell death enhancing pro-survival signaling or synaptic plasticity mediated by NMDAR. Among these modulators, a role is emerging for the enzyme serine racemase (SR) that synthesizes D-serine, a key co-agonist with glutamate at NMDAR. This review summarizes the experimental evidence from "in vitro" neuronal cultures-with special emphasis on cerebellar granule neurons (CGNs)-and "in vivo" models of neurodegeneration, where the dual role of the SR/D-serine pathway as a master regulator of apoptosis and the apoptosis-necrosis shift will be discussed.
Keywords: D-serine; NMDAR; apoptosis-necrosis shift; neurodegeneration; neurological disorders; review; serine racemase.
Figures
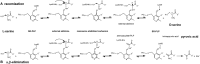
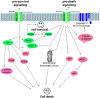
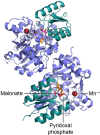
References
-
- Akbulut K. G., Guney S., Cetin F., Agun H. N., Aktas S. H., Akbulut H. (2013). Melatonin delays brain aging by decreasing the nitric oxide level. Neurophysiology 45, 187–192 10.1007/s11062-013-9368-3 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

