β1-Blockers Lower Norepinephrine Release by Inhibiting Presynaptic, Facilitating β1-Adrenoceptors in Normotensive and Hypertensive Rats
- PMID: 24795691
- PMCID: PMC3997042
- DOI: 10.3389/fneur.2014.00051
β1-Blockers Lower Norepinephrine Release by Inhibiting Presynaptic, Facilitating β1-Adrenoceptors in Normotensive and Hypertensive Rats
Abstract
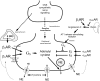
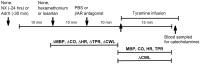
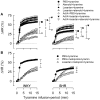
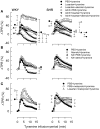
Peripheral norepinephrine release is facilitated by presynaptic β-adrenoceptors, believed to involve the β2-subtype exclusively. However, β1-selective blockers are the most commonly used β-blockers in hypertension. Here the author tested the hypothesis that β1AR may function as presynaptic, release-facilitating auto-receptors. Since β1AR-blockers are injected during myocardial infarction, their influence on the cardiovascular response to acute norepinephrine release was also studied. By a newly established method, using tyramine-stimulated release through the norepinephrine transporter (NET), presynaptic control of catecholamine release was studied in normotensive and spontaneously hypertensive rats. β1AR-selective antagonists (CGP20712A, atenolol, metoprolol) reduced norepinephrine overflow to plasma equally efficient as β2AR-selective (ICI-118551) and β1+2AR (nadolol) antagonists in both strains. Neither antagonist lowered epinephrine secretion. Atenolol, which does not cross the blood-brain barrier, reduced norepinephrine overflow after adrenalectomy (AdrX), AdrX + ganglion blockade, losartan, or nephrectomy. Atenolol and metoprolol reduced resting cardiac work load. During tyramine-stimulated norepinephrine release, they had little effect on work load, and increased the transient rise in total peripheral vascular resistance, particularly atenolol when combined with losartan. In conclusion, β1AR, like β2AR, stimulated norepinephrine but not epinephrine release, independent of adrenal catecholamines, ganglion transmission, or renal renin release/angiotensin AT1 receptor activation. β1AR therefore functioned as a peripheral, presynaptic, facilitating auto-receptor. Like tyramine, hypoxia may induce NET-mediated release. Augmented tyramine-induced vasoconstriction, as observed after injection of β1AR-blocker, particularly atenolol combined with losartan, may hamper organ perfusion, and may have clinical relevance in hypoxic conditions such as myocardial infarction.
Keywords: adrenal glands; angiotensin II, hypertension; atenolol; metoprolol; norepinephrine release; β-adrenoceptors.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

