Non-Inflammasome Forming NLRs in Inflammation and Tumorigenesis
- PMID: 24795716
- PMCID: PMC4001041
- DOI: 10.3389/fimmu.2014.00169
Non-Inflammasome Forming NLRs in Inflammation and Tumorigenesis
Abstract
Aberrant inflammation is an enabling characteristic of tumorigenesis. Thus, signaling cascades that alter inflammatory activation and resolution are of specific relevance to disease pathogenesis. Pattern recognition receptors (PRRs) are essential mediators of the host immune response and have emerged as critical elements affecting multiple facets of tumor pathobiology. The nucleotide-binding domain and leucine-rich repeat containing (NLR) proteins are intracellular PRRs that sense microbial and non-microbial products. Members of the NLR family can be divided into functional sub-groups based on their ability to either positively or negatively regulate the host immune response. Recent studies have identified a novel sub-group of non-inflammasome forming NLRs that negatively regulate diverse biological pathways associated with both inflammation and tumorigenesis. Understanding the mechanisms underlying the function of these unique NLRs will assist in the rationale design of future therapeutic strategies targeting a wide spectrum of inflammatory diseases and cancer. Here, we will discuss recent findings associated with this novel NLR sub-group and mechanisms by which these PRRs may function to alter cancer pathogenesis.
Keywords: NF-κB; NLRC3; NLRP12; NLRX1; Nod-like receptors; TRAF; cancer; pattern recognition receptors.
Figures
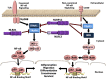

References
-
- Heidland A, Klassen A, Rutkowski P, Bahner U. The contribution of Rudolf Virchow to the concept of inflammation: what is still of importance? J Nephrol (2006) 19(Suppl 10):S102–9 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

