T cell responses to viral infections - opportunities for Peptide vaccination
- PMID: 24795718
- PMCID: PMC3997009
- DOI: 10.3389/fimmu.2014.00171
T cell responses to viral infections - opportunities for Peptide vaccination
Abstract
An effective immune response against viral infections depends on the activation of cytotoxic T cells that can clear infection by killing virus-infected cells. Proper activation of these T cells depends on professional antigen-presenting cells, such as dendritic cells (DCs). In this review, we will discuss the potential of peptide-based vaccines for prevention and treatment of viral diseases. We will describe features of an effective response against both acute and chronic infections, such as an appropriate magnitude, breadth, and quality and discuss requirements for inducing such an effective antiviral immune response. We will address modifications that affect presentation of vaccine components by DCs, including choice of antigen, adjuvants, and formulation. Furthermore, we will describe differences in design between preventive and therapeutic peptide-based vaccines. The ultimate goal in the design of preventive vaccines is to develop a universal vaccine that cross-protects against multiple strains of the virus. For therapeutic vaccines, cross-protection is of less importance, but enhancing existing T cell responses is essential. Although peptide vaccination is successful in inducing responses in human papillomavirus (HPV) infected patients, there are still several challenges such as choosing the right target epitopes, choosing safe adjuvants that improve immunogenicity of these epitopes, and steering the immune response in the desired direction. We will conclude with an overview of the current status of peptide vaccination, hurdles to overcome, and prospects for the future.
Keywords: DC; acute; chronic; infection; peptides; vaccination; virus.
Figures
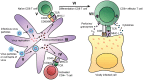
References
-
- Knipe DM, Howley PM. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; (2013).
-
- WHO. Influenza Factsheet. (2009). Available from: http://www.who.int/mediacentre/factsheets/fs211/en/index.html
-
- UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. Geneva: UNAIDS; (2013).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

