When RNA and protein degradation pathways meet
- PMID: 24795741
- PMCID: PMC4006050
- DOI: 10.3389/fpls.2014.00161
When RNA and protein degradation pathways meet
Abstract
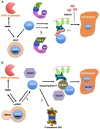
RNA silencing has become a major focus of molecular and biomedical research in the last decade. This mechanism, which is conserved in most eukaryotes, has been extensively studied and is associated to various pathways implicated in the regulation of development, in the control of transposition events, heterochromatin maintenance and also playing a role in defense against viruses. Despite of its importance, the regulation of the RNA silencing machinery itself remains still poorly explored. Recently several reports in both plants and metazoans revealed that key components of RNA silencing, such as RNA-induced silencing complex component ARGONAUTE proteins, but also the endonuclease Dicer are subjected to proteasomal and autophagic pathways. Here we will review these post-translational proteolytic regulations with a special emphasis on plant research and also discuss their functional relevance.
Keywords: RISC; RNA silencing; autophagy; proteasome; ubiquitin; virus.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

