Characterization of genipin-modified dentin collagen
- PMID: 24795891
- PMCID: PMC3984863
- DOI: 10.1155/2014/702821
Characterization of genipin-modified dentin collagen
Abstract
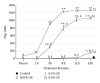
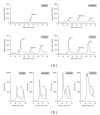
Application of biomodification techniques to dentin can improve its biochemical and biomechanical properties. Several collagen cross-linking agents have been reported to strengthen the mechanical properties of dentin. However, the characteristics of collagen that has undergone agent-induced biomodification are not well understood. The objective of this study was to analyze the effects of a natural cross-linking agent, genipin (GE), on dentin discoloration, collagen stability, and changes in amino acid composition and lysyl oxidase mediated natural collagen cross-links. Dentin collagen obtained from extracted bovine teeth was treated with three different concentrations of GE (0.01%, 0.1%, and 0.5%) for several treatment times (0-24 h). Changes in biochemical properties of NaB(3)H4-reduced collagen were characterized by amino acid and cross-link analyses. The treatment of dentin collagen with GE resulted in a concentration- and time-dependent pigmentation and stability against bacterial collagenase. The lysyl oxidase-mediated trivalent mature cross-link, pyridinoline, showed no difference among all groups while the major divalent immature cross-link, dehydro-dihydroxylysinonorleucine/its ketoamine in collagen treated with 0.5% GE for 24 h, significantly decreased compared to control (P < 0.05). The newly formed GE-induced cross-links most likely involve lysine and hydroxylysine residues of collagen in a concentration-dependent manner. Some of these cross-links appear to be reducible and stabilized with NaB(3)H4.
Figures

Similar articles
-
Site comparisons of dentine collagen cross-links from extracted human teeth.Arch Oral Biol. 1993 Jul;38(7):541-6. doi: 10.1016/0003-9969(93)90118-6. Arch Oral Biol. 1993. PMID: 8368950
-
Glycosylation and cross-linking in bone type I collagen.J Biol Chem. 2014 Aug 15;289(33):22636-22647. doi: 10.1074/jbc.M113.528513. Epub 2014 Jun 23. J Biol Chem. 2014. PMID: 24958722 Free PMC article.
-
Stable, nonreducible cross-links of mature collagen.Biochemistry. 1975 May 6;14(9):2031-6. doi: 10.1021/bi00680a034. Biochemistry. 1975. PMID: 235976
-
Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance.Bone. 1998 Mar;22(3):181-7. doi: 10.1016/s8756-3282(97)00279-2. Bone. 1998. PMID: 9514209 Review.
-
[Progress in biomodification of dentin type Ⅰ collagen by different types of cross-linkers].Zhonghua Kou Qiang Yi Xue Za Zhi. 2020 Feb 9;55(2):135-138. doi: 10.3760/cma.j.issn.1002-0098.2020.02.013. Zhonghua Kou Qiang Yi Xue Za Zhi. 2020. PMID: 32074678 Review. Chinese.
Cited by
-
Stability and remineralization of proteoglycan-infused dentin substrate.Dent Mater. 2021 Nov;37(11):1724-1733. doi: 10.1016/j.dental.2021.09.003. Epub 2021 Sep 15. Dent Mater. 2021. PMID: 34538503 Free PMC article.
-
Evaluation of the effect of collagen stabilizing agents like chitosan and proanthocyanidin on the shear bond strength to dentin and microleakage of resin composite at enamel and cemental walls: An in vitro study.J Conserv Dent. 2019 Sep-Oct;22(5):483-489. doi: 10.4103/JCD.JCD_195_20. Epub 2020 Aug 4. J Conserv Dent. 2019. PMID: 33082667 Free PMC article.
-
Current Trends in Biomaterial Utilization for Cardiopulmonary System Regeneration.Stem Cells Int. 2018 Apr 29;2018:3123961. doi: 10.1155/2018/3123961. eCollection 2018. Stem Cells Int. 2018. PMID: 29853910 Free PMC article. Review.
-
Amnion-derived hydrogels as a versatile platform for regenerative therapy: from lab to market.Front Bioeng Biotechnol. 2024 Feb 26;12:1358977. doi: 10.3389/fbioe.2024.1358977. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38468689 Free PMC article. Review.
-
Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives.Polymers (Basel). 2016 Feb 4;8(2):42. doi: 10.3390/polym8020042. Polymers (Basel). 2016. PMID: 30979136 Free PMC article. Review.
References
-
- Kuboki Y, Mechanic GL. Comparative molecular distribution of cross-links in bone and dentin collagen. Structure-function relationships. Calcified Tissue International. 1982;34(3):306–308. - PubMed
-
- Stetler-Stevenson WG, Veis A. Type I collagen shows a specific binding affinity for bovine dentin phosphophoryn. Calcified Tissue International. 1986;38(3):135–141. - PubMed
-
- Silver FH, Landis WJ. Deposition of apatite in mineralizing vertebrate extracellular matrices: a model of possible nucleation sites on type I collagen. Connective Tissue Research. 2011;52(3):242–254. - PubMed
-
- Nimni M, Harkness R. Molecular structures and functions of collagen. In: Nimni M, editor. Collagen Biochemistry. Boca Raton, Fla, USA: CRC Press; 1988. pp. 1–77.
-
- Yamauchi M, Mechanic GL. Cross-linking of collagen. In: NImni M, Olsen B, editors. Collagen. Boca Raton, Fla, USA: CRC Press; 1988. pp. 157–172.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

