Characterization of genipin-modified dentin collagen
- PMID: 24795891
- PMCID: PMC3984863
- DOI: 10.1155/2014/702821
Characterization of genipin-modified dentin collagen
Abstract
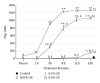
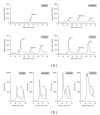
Application of biomodification techniques to dentin can improve its biochemical and biomechanical properties. Several collagen cross-linking agents have been reported to strengthen the mechanical properties of dentin. However, the characteristics of collagen that has undergone agent-induced biomodification are not well understood. The objective of this study was to analyze the effects of a natural cross-linking agent, genipin (GE), on dentin discoloration, collagen stability, and changes in amino acid composition and lysyl oxidase mediated natural collagen cross-links. Dentin collagen obtained from extracted bovine teeth was treated with three different concentrations of GE (0.01%, 0.1%, and 0.5%) for several treatment times (0-24 h). Changes in biochemical properties of NaB(3)H4-reduced collagen were characterized by amino acid and cross-link analyses. The treatment of dentin collagen with GE resulted in a concentration- and time-dependent pigmentation and stability against bacterial collagenase. The lysyl oxidase-mediated trivalent mature cross-link, pyridinoline, showed no difference among all groups while the major divalent immature cross-link, dehydro-dihydroxylysinonorleucine/its ketoamine in collagen treated with 0.5% GE for 24 h, significantly decreased compared to control (P < 0.05). The newly formed GE-induced cross-links most likely involve lysine and hydroxylysine residues of collagen in a concentration-dependent manner. Some of these cross-links appear to be reducible and stabilized with NaB(3)H4.
Figures

References
-
- Kuboki Y, Mechanic GL. Comparative molecular distribution of cross-links in bone and dentin collagen. Structure-function relationships. Calcified Tissue International. 1982;34(3):306–308. - PubMed
-
- Stetler-Stevenson WG, Veis A. Type I collagen shows a specific binding affinity for bovine dentin phosphophoryn. Calcified Tissue International. 1986;38(3):135–141. - PubMed
-
- Silver FH, Landis WJ. Deposition of apatite in mineralizing vertebrate extracellular matrices: a model of possible nucleation sites on type I collagen. Connective Tissue Research. 2011;52(3):242–254. - PubMed
-
- Nimni M, Harkness R. Molecular structures and functions of collagen. In: Nimni M, editor. Collagen Biochemistry. Boca Raton, Fla, USA: CRC Press; 1988. pp. 1–77.
-
- Yamauchi M, Mechanic GL. Cross-linking of collagen. In: NImni M, Olsen B, editors. Collagen. Boca Raton, Fla, USA: CRC Press; 1988. pp. 157–172.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

