Involvement of interleukin-17A-induced hypercontractility of intestinal smooth muscle cells in persistent gut motor dysfunction
- PMID: 24796324
- PMCID: PMC4010403
- DOI: 10.1371/journal.pone.0092960
Involvement of interleukin-17A-induced hypercontractility of intestinal smooth muscle cells in persistent gut motor dysfunction
Abstract
Background and aim: The etiology of post-inflammatory gastrointestinal (GI) motility dysfunction, after resolution of acute symptoms of inflammatory bowel diseases (IBD) and intestinal infection, is largely unknown, however, a possible involvement of T cells is suggested.
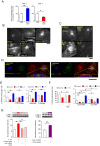
Methods: Using the mouse model of T cell activation-induced enteritis, we investigated whether enhancement of smooth muscle cell (SMC) contraction by interleukin (IL)-17A is involved in postinflammatory GI hypermotility.
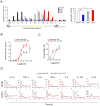
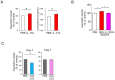
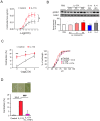
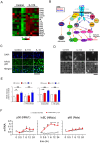
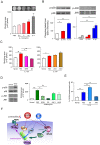
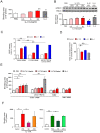
Results: Activation of CD3 induces temporal enteritis with GI hypomotility in the midst of, and hypermotility after resolution of, intestinal inflammation. Prolonged upregulation of IL-17A was prominent and IL-17A injection directly enhanced GI transit and contractility of intestinal strips. Postinflammatory hypermotility was not observed in IL-17A-deficient mice. Incubation of a muscle strip and SMCs with IL-17A in vitro resulted in enhanced contractility with increased phosphorylation of Ser19 in myosin light chain 2 (p-MLC), a surrogate marker as well as a critical mechanistic factor of SMC contractility. Using primary cultured murine and human intestinal SMCs, IκBζ- and p38 mitogen-activated protein kinase (p38MAPK)-mediated downregulation of the regulator of G protein signaling 4 (RGS4), which suppresses muscarinic signaling of contraction by promoting inactivation/desensitization of Gαq/11 protein, has been suggested to be involved in IL-17A-induced hypercontractility. The opposite effect of L-1β was mediated by IκBζ and c-jun N-terminal kinase (JNK) activation.
Conclusions: We propose and discuss the possible involvement of IL-17A and its downstream signaling cascade in SMCs in diarrheal hypermotility in various GI disorders.
Conflict of interest statement
Figures
Similar articles
-
Upregulation of RGS4 expression by IL-1beta in colonic smooth muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the PI3K/Akt/GSK3beta pathway.Am J Physiol Cell Physiol. 2009 Jun;296(6):C1310-20. doi: 10.1152/ajpcell.00573.2008. Epub 2009 Apr 15. Am J Physiol Cell Physiol. 2009. PMID: 19369446 Free PMC article.
-
Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic muscle contraction by interleukin-1beta.Am J Physiol Cell Physiol. 2007 Dec;293(6):C1991-2000. doi: 10.1152/ajpcell.00300.2007. Epub 2007 Oct 24. Am J Physiol Cell Physiol. 2007. PMID: 17959727 Free PMC article.
-
MEKK1-MKK4-JNK-AP1 pathway negatively regulates Rgs4 expression in colonic smooth muscle cells.PLoS One. 2012;7(4):e35646. doi: 10.1371/journal.pone.0035646. Epub 2012 Apr 24. PLoS One. 2012. PMID: 22545125 Free PMC article.
-
IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-alpha and IL-1beta.Am J Physiol Renal Physiol. 2010 Mar;298(3):F779-87. doi: 10.1152/ajprenal.00198.2009. Epub 2009 Dec 30. Am J Physiol Renal Physiol. 2010. PMID: 20042461
-
Interleukin-17A directly acts on bronchial smooth muscle cells and augments the contractility.Pharmacol Rep. 2017 Jun;69(3):377-385. doi: 10.1016/j.pharep.2016.12.007. Epub 2016 Dec 11. Pharmacol Rep. 2017. PMID: 28267638
Cited by
-
Maternal plasma cytokines and the subsequent risk of uterine atony and postpartum hemorrhage.J Perinat Med. 2022 Jun 21;51(2):219-232. doi: 10.1515/jpm-2022-0211. Print 2023 Feb 23. J Perinat Med. 2022. PMID: 35724639 Free PMC article.
-
A novel murine model of autoimmune dysautonomia by α3 nicotinic acetylcholine receptor immunization.Front Neurosci. 2022 Nov 23;16:1006923. doi: 10.3389/fnins.2022.1006923. eCollection 2022. Front Neurosci. 2022. PMID: 36507326 Free PMC article.
-
Crosstalk at the mucosal border: importance of the gut microenvironment in IBS.Nat Rev Gastroenterol Hepatol. 2015 Jan;12(1):36-49. doi: 10.1038/nrgastro.2014.200. Epub 2014 Dec 2. Nat Rev Gastroenterol Hepatol. 2015. PMID: 25446728 Review.
-
Genome analysis identifies differences in the transcriptional targets of duodenal versus pancreatic neuroendocrine tumours.BMJ Open Gastroenterol. 2021 Nov;8(1):e000765. doi: 10.1136/bmjgast-2021-000765. BMJ Open Gastroenterol. 2021. PMID: 34750164 Free PMC article.
-
The Role of Inflammatory Mediators in the Development of Gastrointestinal Motility Disorders.Int J Mol Sci. 2022 Jun 22;23(13):6917. doi: 10.3390/ijms23136917. Int J Mol Sci. 2022. PMID: 35805922 Free PMC article. Review.
References
-
- Brand S (2009) Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut 58: 1152–1167. - PubMed
-
- Facco M, Brun P, Baesso I, Costantini M, Rizzetto C, et al. (2008) T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol 103: 1598–1609. - PubMed
-
- Frasko R, Maruna P, Gurlich R, Trca S (2008) Transcutaneous electrogastrography in patients with ileus. Relations to interleukin-1beta, interleukin-6, procalcitonin and C-reactive protein. Eur Surg Res 41: 197–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

