Pharmacokinetics and efficacy of topically applied nonsteroidal anti-inflammatory drugs in retinochoroidal tissues in rabbits
- PMID: 24796327
- PMCID: PMC4010472
- DOI: 10.1371/journal.pone.0096481
Pharmacokinetics and efficacy of topically applied nonsteroidal anti-inflammatory drugs in retinochoroidal tissues in rabbits
Abstract
Purpose: To evaluate the pharmacokinetics and efficacy of topically applied nonsteroidal anti-inflammatory drugs (NSAIDs) in the retinochoroidal tissues of rabbits.
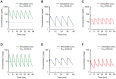
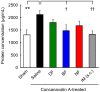
Methods: The cyclooxygenase (COX) inhibitory activity of diclofenac, bromfenac, and amfenac, an active metabolite of nepafenac, were determined using human-derived COX-1 and COX-2. Each of the three NSAIDs was applied topically to rabbits, and after 0.5 to 8 hrs, the concentration of each drug in the aqueous humor and the retinochoroidal tissues was measured by liquid chromatography-tandem mass spectrometry. The pharmacokinetics of the drugs in the tissues after repeated doses as is done on patients was calculated by a simulation software. The inhibitory effect of each NSAID on the breakdown of the blood-retinal barrier was assessed by the vitreous protein concentration on concanavalin A-induced retinochoroidal inflammation in rabbits.
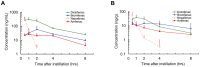
Results: The half-maximal inhibitory concentration (IC50) of diclofenac, bromfenac, and amfenac was 55.5, 5.56, and 15.3 nM for human COX-1, and 30.7, 7.45, and 20.4 nM for human COX-2, respectively. The three NSAIDs were detected in the aqueous humor and the retinochoroidal tissue at all-time points. Simulated pharmacokinetics showed that the levels of the three NSAIDs were continuously higher than the IC50 of COX-2, as an index of efficacy, in the aqueous humor, whereas only the bromfenac concentration was continuously higher than the IC50 at its trough level in the retinochoroidal tissues. The intravitreous concentration of proteins was significantly reduced in rabbits that received topical bromfenac (P = 0.026) but not the other two NSAIDs.
Conclusions: Topical bromfenac can penetrate into the retinochoroidal tissues in high enough concentrations to inhibit COX-2 and exerts its inhibitory effect on the blood-retinal barrier breakdown in an experimental retinochoroidal inflammation in rabbits. Topical bromfenac may have a better therapeutic benefit than diclofenac and nepafenac for retinochoroidal inflammatory diseases.
Conflict of interest statement
Figures
Similar articles
-
In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac.J Cataract Refract Surg. 2007 Sep;33(9):1539-45. doi: 10.1016/j.jcrs.2007.05.015. J Cataract Refract Surg. 2007. PMID: 17720067 Clinical Trial.
-
VITREOUS PROSTAGLANDIN E2 CHANGES AFTER TOPICAL ADMINISTRATION OF DICLOFENAC 0.1%, INDOMETHACIN 0.5%, NEPAFENAC 0.3%, AND BROMFENAC 0.09.Retina. 2020 Sep;40(9):1838-1845. doi: 10.1097/IAE.0000000000002674. Retina. 2020. PMID: 31800462 Clinical Trial.
-
A randomized comparison of to-aqueous penetration of ketorolac 0.45%, bromfenac 0.09% and nepafenac 0.1% in cataract patients undergoing phacoemulsification.Curr Med Res Opin. 2011 Dec;27(12):2235-9. doi: 10.1185/03007995.2011.626018. Epub 2011 Oct 12. Curr Med Res Opin. 2011. PMID: 21992076 Clinical Trial.
-
Nepafenac: an ophthalmic nonsteroidal antiinflammatory drug for pain after cataract surgery.Ann Pharmacother. 2013 Jun;47(6):892-6. doi: 10.1345/aph.1R757. Epub 2013 May 28. Ann Pharmacother. 2013. PMID: 23715071 Review.
-
Ocular permeation and inhibition of retinal inflammation: an examination of data and expert opinion on the clinical utility of nepafenac.Curr Med Res Opin. 2006 Feb;22(2):397-404. doi: 10.1185/030079906X89775. Curr Med Res Opin. 2006. PMID: 16466612 Review.
Cited by
-
Repurposing High-Throughput Screening Reveals Unconventional Drugs with Antimicrobial and Antibiofilm Potential Against Methicillin-Resistant Staphylococcus aureus from a Cystic Fibrosis Patient.Antibiotics (Basel). 2025 Apr 14;14(4):402. doi: 10.3390/antibiotics14040402. Antibiotics (Basel). 2025. PMID: 40298549 Free PMC article.
-
Text Mining-Based Drug Discovery in Osteoarthritis.J Healthc Eng. 2021 Apr 14;2021:6674744. doi: 10.1155/2021/6674744. eCollection 2021. J Healthc Eng. 2021. PMID: 33953899 Free PMC article.
-
Topical bromfenac reduces multiple inflammatory cytokines in the aqueous humour of pseudophakic patients.Sci Rep. 2021 Mar 16;11(1):6018. doi: 10.1038/s41598-021-85495-w. Sci Rep. 2021. PMID: 33727659 Free PMC article. Clinical Trial.
-
An ex vivo human aqueous humor-concentration comparison of two commercial bromfenac formulations.Clin Ophthalmol. 2018 May 21;12:943-947. doi: 10.2147/OPTH.S170540. eCollection 2018. Clin Ophthalmol. 2018. PMID: 29849449 Free PMC article.
-
Topical bromfenac for prevention and treatment of cystoid macular edema following cataract surgery: a review.Clin Ophthalmol. 2016 Oct 25;10:2099-2111. doi: 10.2147/OPTH.S86971. eCollection 2016. Clin Ophthalmol. 2016. PMID: 27822006 Free PMC article. Review.
References
-
- Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya'ale D, et al. (2004) 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol 11: 67–115. - PubMed
-
- Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, et al. (2004) Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 122: 477–485. - PubMed
-
- Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, et al. (2004) Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 122: 564–572. - PubMed
-
- Miller JW, Le Couter J, Strauss EC, Ferrara N (2013) Vascular endothelial growth factor A in intraocular vascular disease. Ophthalmology 120: 106–114. - PubMed
-
- Do DV (2013) Implications of the comparisons of age-related macular degeneration treatments trials on clinical practice: what have we learned? Ophthalmology 120: S8–S10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

