Voice disorder in cystic fibrosis patients
- PMID: 24796691
- PMCID: PMC4010511
- DOI: 10.1371/journal.pone.0096769
Voice disorder in cystic fibrosis patients
Abstract
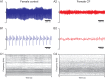
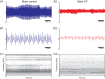
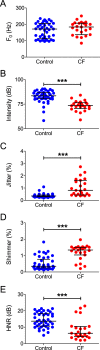
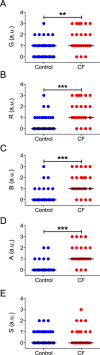
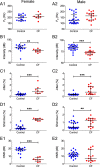
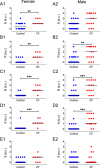
Cystic fibrosis is a common autosomal recessive disorder with drastic respiratory symptoms, including shortness of breath and chronic cough. While most of cystic fibrosis treatment is dedicated to mitigating the effects of respiratory dysfunction, the potential effects of this disease on vocal parameters have not been systematically studied. We hypothesized that cystic fibrosis patients, given their characteristic respiratory disorders, would also present dysphonic symptoms. Given that voice disorders can severely impair quality of life, the identification of a potential cystic fibrosis-related dysphonia could be of great value for the clinical evaluation and treatment of this disease. We tested our hypothesis by measuring vocal parameters, using both objective physical measures and the GRBAS subjective evaluation method, in male and female cystic fibrosis patients undergoing conventional treatment and compared them to age and sex matched controls. We found that cystic fibrosis patients had a significantly lower vocal intensity and harmonic to noise ratio, as well as increased levels of jitter and shimmer. In addition, cystic fibrosis patients also showed higher scores of roughness, breathiness and asthenia, as well as a significantly altered general grade of dysphonia. When we segregated the results according to sex, we observed that, as a group, only female cystic fibrosis patients had significantly lower values of harmonic to noise ratio and an abnormal general grade of dysphonia in relation to matched controls, suggesting that cystic fibrosis exerts a more pronounced effect on vocal parameters of women in relation to men. Overall, the dysphonic characteristics of CF patients can be explained by dysfunctions in vocal fold movement and partial upper airway obstruction, potentially caused by the accumulation of mucus and chronic cough characteristic of CF symptomatology. Our results show that CF patients exhibit significant dysphonia and suggest they may potentially benefit from voice therapy as a parallel treatment strategy.
Conflict of interest statement
Figures
References
-
- Wilson JA, Deary IJ, Millar A, Mackenzie K (2002) The quality of life impact of dysphonia. Clin Otolaryngol Allied Sci 27: 179–182. - PubMed
-
- Deary IJ, Scott S, Wilson IM, White A, MacKenzie K, et al. (1997) Personality and psychological distress in dysphonia. Br J Health Psychol 2: 333–341 10.1111/j.2044-8287.1997.tb00547.x - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

