A randomized clinical trial to determine optimal infertility treatment in older couples: the Forty and Over Treatment Trial (FORT-T)
- PMID: 24796764
- PMCID: PMC4106674
- DOI: 10.1016/j.fertnstert.2014.03.012
A randomized clinical trial to determine optimal infertility treatment in older couples: the Forty and Over Treatment Trial (FORT-T)
Abstract
Objective: To determine the optimal infertility therapy for women at the end of their reproductive potential.
Design: Randomized clinical trial.
Setting: Academic medical centers and private infertility center in a state with mandated insurance coverage.
Patient(s): Couples with ≥ 6 months of unexplained infertility; female partner aged 38-42 years.
Intervention(s): Randomized to treatment with two cycles of clomiphene citrate (CC) and intrauterine insemination (IUI), follicle stimulating hormone (FSH)/IUI, or immediate IVF, followed by IVF if not pregnant.
Main outcome measure(s): Proportion with a clinically recognized pregnancy, number of treatment cycles, and time to conception after two treatment cycles and at the end of treatment.
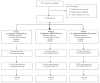
Result(s): We randomized 154 couples to receive CC/IUI (N = 51), FSH/IUI (N = 52), or immediate IVF (N = 51); 140 (90.9%) couples initiated treatment. The cumulative clinical pregnancy rates per couple after the first two cycles of CC/IUI, FSH/IUI, or immediate IVF were 21.6%, 17.3%, and 49.0%, respectively. After all treatments, 110 (71.4%) of 154 couples had conceived a clinically recognized pregnancy, and 46.1% had delivered at least one live-born baby; 84.2% of all live-born infants resulting from treatment were achieved via IVF. There were 36% fewer treatment cycles in the IVF arm compared with either COH/IUI arm, and the couples conceived a pregnancy leading to a live birth after fewer treatment cycles.
Conclusion(s): A randomized controlled trial in older women with unexplained infertility to compare treatment initiated with two cycles of controlled ovarian hyperstimulation/IUI versus immediate IVF demonstrated superior pregnancy rates with fewer treatment cycles in the immediate IVF group.
Clinical trial registration number: NCT00246506.
Keywords: Advanced reproductive age; FORT-T Trial; clomiphene citrate; controlled ovarian hyperstimulation; follicle-stimulating hormone; intrauterine insemination (IUI); in vitro fertilization; unexplained infertility.
Copyright © 2014 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Insights on managing the older couple with unexplained infertility and normal ovarian reserve.Fertil Steril. 2014 Jun;101(6):1586-7. doi: 10.1016/j.fertnstert.2014.03.028. Epub 2014 Apr 30. Fertil Steril. 2014. PMID: 24794317 No abstract available.
References
-
- Guzick DS, Sullivan MW, Adamson GD, Cedars MI, Falk RJ, Peterson EP, et al. Efficacy of treatment for unexplained infertility. Fertil Steril. 1998;70:207–213. - PubMed
-
- Guzick DS, Carson SA, Coutifaris C, Overstreet JW, Factor-Litvak P, Steinkampf MP, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. N Engl J Med. 1999;340:177–183. - PubMed
-
- McClamrock HD, Jones HW, Adashi EY. Ovarian stimulation and intrauterine insemination at the quarter centennial: implications for the multiple births epidemic. Fertil Steril. 2012;97:802–809. - PubMed
-
- Reindollar RH, Regan MM, Neumann PJ, Levine B, Thornton KL, Alper MM, Goldman MB. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94:888–899. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

