First multicenter study of modified release phosphatidylcholine "LT-02" in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses
- PMID: 24796768
- PMCID: PMC4085478
- DOI: 10.1038/ajg.2014.104
First multicenter study of modified release phosphatidylcholine "LT-02" in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses
Abstract
Objectives: Phosphatidylcholine is a key component of the mucosal barrier. Treatment with modified release phosphatidylcholine aims to improve the impaired barrier function. The primary objective is to evaluate the efficacy of LT-02, a newly designed modified release phosphatidylcholine formula, in a multicenter setting.
Methods: This is a double-blinded, randomized, placebo-controlled, superiority study conducted in 24 ambulatory referral centers in Germany, Lithuania, and Romania. A total of 156 patients with an inadequate response to mesalazine, a disease activity score (Simple Clinical Colitis Activity Index (SCCAI)) of ≥ 5, and bloody diarrhea underwent treatment with 0, 0.8, 1.6, or 3.2 g LT-02. The primary end point was defined a priori as changes in SCCAI from baseline to the end of treatment. The primary statistical model was a general linear least-squares model. The study was funded by the sponsor Lipid Therapeutics, Heidelberg, Germany, and registered at http://clinicaltrials.gov/show/NCT01011322.
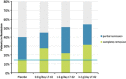
Results: Baseline characteristics and dropouts were well balanced between all groups. The primary analyses revealed an SCCAI drop of 33.3% in the placebo group (from 9.0 to 6.0 points) compared with 44.3% in the 0.8 g LT-02 (from 8.8 to 4.9, P>0.05) and 40.7% in the 1.6 g groups (from 8.6 to 5.1, P>0.05). The 3.2 g group improved 51.7% from 8.5 to 4.1 (P=0.030 in comparison with placebo). The remission rate was 15% (6/40) in the placebo group compared with 31.4% (11/35) in the highest LT-02 dose group (P=0.089). Mucosal healing was achieved in 32.5% of placebo patients compared with 47.4% of LT-02 patients (P=0.098); the rates for histologic remission were 20% compared with 40.5%, respectively (P=0.016). There were 17 (48.6%) treatment-emergent adverse events in the highest dose group (and 0 serious adverse events (SAEs)) compared with 22 (55%) in the placebo group (4 SAEs).
Conclusions: The primary end point analysis showed a statistically significant improvement in disease activity during LT-02 treatment in comparison with placebo. The drug was found to be very safe.
Figures
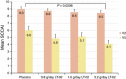
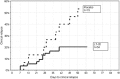
 The comparison between placebo and the highest dose group revealed an estimate of −1.56 and a two-sided P value of 0.03 with a 95% confidence interval of −2.96 to −0.16. SCCAI, Simple Clinical Colitis Activity Index.
The comparison between placebo and the highest dose group revealed an estimate of −1.56 and a two-sided P value of 0.03 with a 95% confidence interval of −2.96 to −0.16. SCCAI, Simple Clinical Colitis Activity Index.

Comment in
-
The IBD drug pipeline-ready to deliver?Am J Gastroenterol. 2014 Jul;109(7):1052-4. doi: 10.1038/ajg.2014.179. Am J Gastroenterol. 2014. PMID: 24989095
References
-
- Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. - PubMed
-
- Baumgart DC, Macdonald JK, Feagan B. Tacrolimus (FK506) for induction of remission in refractory ulcerative colitis. Cochrane Database Syst Rev. 2008. p. CD007216. - PubMed
-
- De Vries HS, van Oijen MG, de Jong DJ. Serious events with infliximab in patients with inflammatory bowel disease: a 9-year cohort study in the Netherlands. Drug Saf. 2008;31:1135–1144. - PubMed
-
- Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-center cohort study. Gut. 2009;58:501–508. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

