Glucose controls morphodynamics of LPS-stimulated macrophages
- PMID: 24796786
- PMCID: PMC4010488
- DOI: 10.1371/journal.pone.0096786
Glucose controls morphodynamics of LPS-stimulated macrophages
Abstract
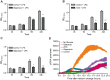
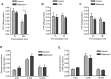
Macrophages constantly undergo morphological changes when quiescently surveying the tissue milieu for signs of microbial infection or damage, or after activation when they are phagocytosing cellular debris or foreign material. These morphofunctional alterations require active actin cytoskeleton remodeling and metabolic adaptation. Here we analyzed RAW 264.7 and Maf-DKO macrophages as models to study whether there is a specific association between aspects of carbohydrate metabolism and actin-based processes in LPS-stimulated macrophages. We demonstrate that the capacity to undergo LPS-induced cell shape changes and to phagocytose complement-opsonized zymosan (COZ) particles does not depend on oxidative phosphorylation activity but is fueled by glycolysis. Different macrophage activities like spreading, formation of cell protrusions, as well as phagocytosis of COZ, were thereby strongly reliant on the presence of low levels of extracellular glucose. Since global ATP production was not affected by rewiring of glucose catabolism and inhibition of glycolysis by 2-deoxy-D-glucose and glucose deprivation had differential effects, our observations suggest a non-metabolic role for glucose in actin cytoskeletal remodeling in macrophages, e.g. via posttranslational modification of receptors or signaling molecules, or other effects on the machinery that drives actin cytoskeletal changes. Our findings impute a decisive role for the nutrient state of the tissue microenvironment in macrophage morphodynamics.
Conflict of interest statement
Figures




References
-
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM (2000) M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J Immunol 164: 6166–6173.
-
- Mantovani A, Sica A, Locati M (2005) Macrophage Polarization Comes of Age. Immunity 23: 344–346. - PubMed
-
- Rodríguez-Prados J-C, Través PG, Cuenca J, Rico D, Aragonés J, et al. (2010) Substrate Fate in Activated Macrophages: A Comparison between Innate, Classic, and Alternative Activation. J Immunol 185: 605–614. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

