Functional amyloids in the mouse sperm acrosome
- PMID: 24797071
- PMCID: PMC4097662
- DOI: 10.1128/MCB.00073-14
Functional amyloids in the mouse sperm acrosome
Abstract
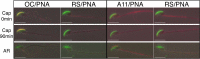
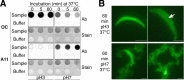
The acrosomal matrix (AM) is an insoluble structure within the sperm acrosome that serves as a scaffold controlling the release of AM-associated proteins during the sperm acrosome reaction. The AM also interacts with the zona pellucida (ZP) that surrounds the oocyte, suggesting a remarkable stability that allows its survival despite being surrounded by proteolytic and hydrolytic enzymes released during the acrosome reaction. To date, the mechanism responsible for the stability of the AM is not known. Our studies demonstrate that amyloids are present within the sperm AM and contribute to the formation of an SDS- and formic-acid-resistant core. The AM core contained several known amyloidogenic proteins, as well as many proteins predicted to form amyloid, including several ZP binding proteins, suggesting a functional role for the amyloid core in sperm-ZP interactions. While stable at pH 3, at pH 7, the sperm AM rapidly destabilized. The pH-dependent dispersion of the AM correlated with a change in amyloid structure leading to a loss of mature forms and a gain of immature forms, suggesting that the reversal of amyloid is integral to AM dispersion.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
