STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis
- PMID: 24797475
- PMCID: PMC4198027
- DOI: 10.1002/embj.201387329
STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis
Abstract
Cohesion between sister chromatids in mitotic and meiotic cells is promoted by a ring-shaped protein structure, the cohesin complex. The cohesin core complex is composed of four subunits, including two structural maintenance of chromosome (SMC) proteins, one α-kleisin protein, and one SA protein. Meiotic cells express both mitotic and meiosis-specific cohesin core subunits, generating cohesin complexes with different subunit composition and possibly separate meiotic functions. Here, we have analyzed the in vivo function of STAG3, a vertebrate meiosis-specific SA protein. Mice with a hypomorphic allele of Stag3, which display a severely reduced level of STAG3, are viable but infertile. We show that meiocytes in homozygous mutant Stag3 mice display chromosome axis compaction, aberrant synapsis, impaired recombination and developmental arrest. We find that the three different α-kleisins present in meiotic cells show different dosage-dependent requirements for STAG3 and that STAG3-REC8 cohesin complexes have a critical role in supporting meiotic chromosome structure and functions.
Keywords: chromosome; cohesin; meiosis; recombination; synaptonemal complex.
© 2014 The Authors.
Figures


Structure of the Stag3 mutant allele. The transgene (LV2229) is inserted within the intron between exon 8 and exon 9 of the Stag3 gene. The positions of PCR primers for genotyping are indicated.
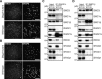
PCR analysis for genotyping. Genomic DNA from wild-type, heterozygous mutant, and homozygous mutant mice were analyzed using the primer pairs indicated in (A).
RT-PCR analysis of RNA extracted from testes of wild-type, heterozygous mutant, and homozygous mutant mice. The signals were quantified, and relative values for Stag3 mRNA abundance normalized to Actin are presented below the panels.
Immunoblotting analysis of testicular protein extracts from wild-type, heterozygous mutant, and homozygous mutant mice. The signals were quantified, and relative values for STAG3 normalized to actin are presented below the panels.
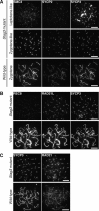
Immunostaining of wild-type and homozygous Stag3 mutant spermatocytes and oocytes. Nuclear spreads were stained for SYCP3 (an AE protein labeling the meiotic chromosome axis) and STAG3. High-exposure images of STAG3 staining (high) are shown to highlight residual STAG3 in the mutant. Bars, 10 μm.
Testes from 8-week-old wild-type, heterozygous Stag3 mutant, and homozygous Stag3 mutant mice.
Testicular histology of 8-week-old wild-type and homozygous Stag3 mutant mice. Testis sections were stained with hematoxylin and eosin. Bars, 100 μm.
Ovaries and oviducts from 8-week-old wild-type and homozygous Stag3 mutant mice.
Ovarian histology of 8-week-old wild-type and homozygous Stag3 mutant mice. Ovary sections were stained with hematoxylin and eosin. Bars, 100 μm.

Immunostaining of wild-type and homozygous Stag3 mutant spermatocytes for SMC3, SYCP2, and SYCP3. Bars, 10 μm.
Immunostaining of wild-type and homozygous Stag3 mutant spermatocytes for REC8, RAD21L, and SYCP2. Bars, 10 μm.
Immunostaining of wild-type and homozygous Stag3 mutant spermatocytes for SYCP3 and RAD21. Bars, 10 μm.
Immunoblotting analysis of testicular protein extracts from wild-type, heterozygous Stag3 mutant, and homozygous Stag3 mutant mice.
Testicular protein extracts were separated into cytoplasmic (Cyto) and nuclear fractions. The nuclear fraction was further fractionated into soluble (Nuc, S) and insoluble (Nuc, P) fractions.
Immunostaining of wild-type and homozygous Stag3 mutant E16.5 oocytes for SMC3, SYCP2, and SYCP3. Bars, 10 μm.
Immunostaining of wild-type and homozygous Stag3 mutant E16.5 oocytes for REC8, RAD21L and SYCP3. Bars, 10 μm.
Immunostaining of wild-type and homozygous Stag3 mutant E16.5 oocytes for SYCP3 and RAD21. Bars, 10 μm.
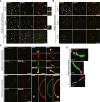
Immunostaining of homozygous Stag3 mutant spermatocytes for SYCP2 and SYCP1. Enlarged views of the axes (arrowheads) are shown to the right. Bars, 10 μm.
Immunostaining of homozygous Stag3 mutant E16.5 and E18.5 oocytes for SYCP3 and SYCP1. Bars, 10 μm.
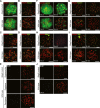
SIM analysis of wild-type and homozygous Stag3 mutant spermatocytes immunostained for SYCP3, SYCE1, and ACA. Enlarged views of axial structures from mutant spermatocytes and homologous chromosomes from wild-type spermatocytes are shown to the right. Scale bars are 10 μm and 1 μm for whole and close-up images, respectively.
Immunostaining of homozygous Stag3 mutant E18.5 oocytes for SYCP3 and ACA displaying different types of aberrant chromosome structures.
References
-
- Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC. Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis. 2004;40:184–194. - PubMed
-
- Costa Y, Speed R, Ollinger R, Alsheimer M, Semple CA, Gautier P, Maratou K, Novak I, Hoog C, Benavente R, Cooke HJ. Two novel proteins recruited by synaptonemal complex protein 1 (SYCP1) are at the centre of meiosis. J Cell Sci. 2005;118:2755–2762. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

