Poldip2 knockout results in perinatal lethality, reduced cellular growth and increased autophagy of mouse embryonic fibroblasts
- PMID: 24797518
- PMCID: PMC4010529
- DOI: 10.1371/journal.pone.0096657
Poldip2 knockout results in perinatal lethality, reduced cellular growth and increased autophagy of mouse embryonic fibroblasts
Abstract
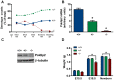
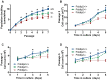
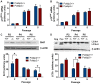
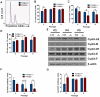
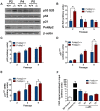
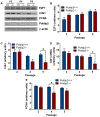
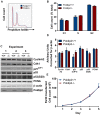
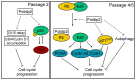
Polymerase-δ interacting protein 2 (Poldip2) is an understudied protein, originally described as a binding partner of polymerase delta and proliferating cell nuclear antigen (PCNA). Numerous roles for Poldip2 have been proposed, including mitochondrial elongation, DNA replication/repair and ROS production via Nox4. In this study, we have identified a novel role for Poldip2 in regulating the cell cycle. We used a Poldip2 gene-trap mouse and found that homozygous animals die around the time of birth. Poldip2-/- embryos are significantly smaller than wild type or heterozygous embryos. We found that Poldip2-/- mouse embryonic fibroblasts (MEFs) exhibit reduced growth as measured by population doubling and growth curves. This effect is not due to apoptosis or senescence; however, Poldip2-/- MEFs have higher levels of the autophagy marker LC3b. Measurement of DNA content by flow cytometry revealed an increase in the percentage of Poldip2-/- cells in the G1 and G2/M phases of the cell cycle, accompanied by a decrease in the percentage of S-phase cells. Increases in p53 S20 and Sirt1 were observed in passage 2 Poldip2-/- MEFs. In passage 4/5 MEFs, Cdk1 and CyclinA2 are downregulated in Poldip2-/- cells, and these changes are reversed by transfection with SV40 large T-antigen, suggesting that Poldip2 may target the E2F pathway. In contrast, p21CIP1 is increased in passage 4/5 Poldip2-/- MEFs and its expression is unaffected by SV40 transfection. Overall, these results reveal that Poldip2 is an essential protein in development, and underline its importance in cell viability and proliferation. Because it affects the cell cycle, Poldip2 is a potential novel target for treating proliferative conditions such as cancer, atherosclerosis and restenosis.
Conflict of interest statement
Figures

References
-
- Liu L, Rodriguez-Belmonte EM, Mazloum N, Xie B, Lee MY (2003) Identification of a novel protein, PDIP38, that interacts with the p50 subunit of DNA polymerase delta and proliferating cell nuclear antigen. J Biol Chem 278: 10041–10047. - PubMed
-
- Klaile E, Kukalev A, Obrink B, Muller MM (2008) PDIP38 is a novel mitotic spindle-associated protein that affects spindle organization and chromosome segregation. Cell Cycle 7: 3180–3186. - PubMed
-
- Tissier A, Janel-Bintz R, Coulon S, Klaile E, Kannouche P, et al. (2010) Crosstalk between replicative and translesional DNA polymerases: PDIP38 interacts directly with Poleta. DNA Repair (Amst) 9: 922–928. - PubMed
-
- Cheng X, Kanki T, Fukuoh A, Ohgaki K, Takeya R, et al. (2005) PDIP38 associates with proteins constituting the mitochondrial DNA nucleoid. J Biochem 138: 673–678. - PubMed
-
- Arakaki N, Nishihama T, Kohda A, Owaki H, Kuramoto Y, et al. (2006) Regulation of mitochondrial morphology and cell survival by Mitogenin I and mitochondrial single-stranded DNA binding protein. Biochim Biophys Acta 1760: 1364–1372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

