Phorbol myristate acetate, but not CD40L, induces the differentiation of CLL B cells into Ab-secreting cells
- PMID: 24797583
- PMCID: PMC4134517
- DOI: 10.1038/icb.2014.37
Phorbol myristate acetate, but not CD40L, induces the differentiation of CLL B cells into Ab-secreting cells
Abstract
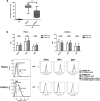
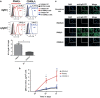
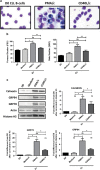
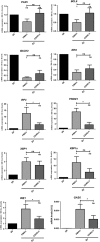
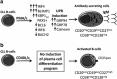
In this study, we investigated the capacity of chronic lymphocytic leukemia (CLL) B cells to undergo terminal differentiation into Ig-secreting plasma cells in T cell-independent and T cell-dependent responses. We used a two-step model involving stimulation with phorbol myristate acetate (PMA) and CD40L, together with cytokines (PMA/c and CD40L/c), for 7 days. We describe immunophenotypic modifications, changes in the levels of mRNA and protein for transcription factors and morphological and functional events occurring during the differentiation of CLL B cells into antibody-secreting cells (ASCs). The induction of differentiation differed significantly between the CD40L/c and PMA/c culture systems. The PMA/c culture system allowed CLL B cells to differentiate into IgM-secreting cells with an immunophenotype and molecular profile resembling those of preplasmablasts. By contrast, CD40L/c-stimulated cells had a phenotype and morphology similar to those of activated B cells and resembling those of the CLL B cells residing in the lymph node and bone marrow. These data suggest that the CLL B cells are not frozen permanently at a stage of differentiation and are able to differentiate into ASCs as appropriate stimulation are provided. The data presented here raise questions about the molecular processes and stimulation required for CLL B-cell differentiation and about the inability of CD40 ligand to induce differentiation of the CLL B cells.
Figures



References
-
- Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. - PubMed
-
- Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. - PubMed
-
- Nera KP, Lassila O. Pax5—a critical inhibitor of plasma cell fate. Scand J Immunol. 2006;64:190–199. - PubMed
-
- Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

