Synthesis and reactivity of 4'-deoxypentenosyl disaccharides
- PMID: 24797640
- PMCID: PMC4059249
- DOI: 10.1021/jo500449h
Synthesis and reactivity of 4'-deoxypentenosyl disaccharides
Abstract
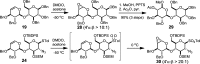
4-Deoxypentenosides (4-DPs) are versatile synthons for rare or higher-order pyranosides, and they provide an entry for structural diversification at the C5 position. Previous studies have shown that 4-DPs undergo stereocontrolled DMDO oxidation; subsequent epoxide ring-openings with various nucleophiles can proceed with both anti or syn selectivity. Here, we report the synthesis of α- and β-linked 4'-deoxypentenosyl (4'-DP) disaccharides, and we investigate their post-glycosylational C5' additions using the DMDO oxidation/ring-opening sequence. The α-linked 4'-DP disaccharides were synthesized by coupling thiophenyl 4-DP donors with glycosyl acceptors using BSP/Tf2O activation, whereas β-linked 4'-DP disaccharides were generated by the decarboxylative elimination of glucuronyl disaccharides under microwave conditions. Both α- and β-linked 4'-DP disaccharides could be epoxidized with high stereoselectivity using DMDO. In some cases, the α-epoxypentenosides could be successfully converted into terminal l-iduronic acids via the syn addition of 2-furylzinc bromide. These studies support a novel approach to oligosaccharide synthesis, in which the stereochemical configuration of the terminal 4'-DP unit is established at a post-glycosylative stage.
Figures









References
-
- Smoot J. T.; Demchenko A. V. Adv. Carbohydr. Chem. Biochem. 2009, 62, 161–250. - PubMed
-
- Zhu X.; Schmidt R. R. Angew. Chem., Int. Ed. 2009, 48, 1900–1934. - PubMed
-
- Crich D. Acc. Chem. Res. 2010, 43, 1144–1153. - PubMed
-
- Liew S.-T.; Wei A. Carbohydr. Res. 2002, 337, 1319–1324. - PubMed
-
- Lohman G. J. S.; Seeberger P. H. J. Org. Chem. 2004, 69, 4081–4093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

