Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats
- PMID: 24797666
- PMCID: PMC4010527
- DOI: 10.1371/journal.pone.0096547
Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats
Abstract
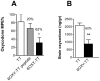
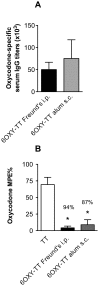
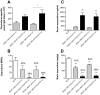
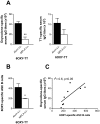
Vaccination against the highly abused prescription opioid oxycodone has shown pre-clinical efficacy for blocking oxycodone effects. The current study further evaluated a candidate vaccine composed of oxycodone derivatized at the C6 position (6OXY) conjugated to the native keyhole limpet hemocyanin (nKLH) carrier protein. To provide an oxycodone vaccine formulation suitable for human studies, we studied the effect of alternative carriers and adjuvants on the generation of oxycodone-specific serum antibody and B cell responses, and the effect of immunization on oxycodone distribution and oxycodone-induced antinociception in mice and rats. 6OXY conjugated to tetanus toxoid (TT) or a GMP grade KLH dimer (dKLH) was as effective as 6OXY conjugated to the nKLH decamer in mice and rats, while the 6OXY hapten conjugated to a TT-derived peptide was not effective in preventing oxycodone-induced antinociception in mice. Immunization with 6OXY-TT s.c. absorbed on alum adjuvant provided similar protection to 6OXY-TT administered i.p. with Freund's adjuvant in rats. The toll-like receptor 4 (TLR4) agonist monophosphoryl lipid A (MPLA) adjuvant, alone or in combination with alum, offered no advantage over alum alone for generating oxycodone-specific serum antibodies or 6OXY-specific antibody secreting B cells in mice vaccinated with 6OXY-nKLH or 6OXY-TT. The immunogenicity of oxycodone vaccines may be modulated by TLR4 signaling since responses to 6OXY-nKLH in alum were decreased in TLR4-deficient mice. These data suggest that TT, nKLH and dKLH carriers provide consistent 6OXY conjugate vaccine immunogenicity across species, strains and via different routes of administration, while adjuvant formulations may need to be tailored to individual immunogens or patient populations.
Conflict of interest statement
Figures



References
-
- UNODC (2012) World Drug Report 2012.
-
- National Survey on Drug Use and Health: National Findings (2013)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

