A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a Sarcoma Alliance for Research Through Collaboration study
- PMID: 24797726
- PMCID: PMC7009782
- DOI: 10.1002/cncr.28728
A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a Sarcoma Alliance for Research Through Collaboration study
Abstract
Background: Insulin-like growth factor-1 receptor (IGF-1R) is implicated in the pathogenesis of rhabdomyosarcoma (RMS), osteosarcoma (OS), and synovial sarcoma (SS). The authors conducted a multi-institutional phase 2 trial of the monoclonal antibody R1507 in patients with various subtypes of recurrent or refractory sarcomas.
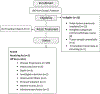
Methods: Eligibility criteria included age ≥ 2 years and a diagnosis of recurrent or refractory RMS, OS, SS, and other soft tissue sarcomas. Patients received a weekly dose of 9 mg/kg R1507 intravenously. The primary endpoint was the best objective response rate using World Health Organization criteria. Tumor imaging was performed every 6 weeks × 4 and every 12 weeks thereafter.
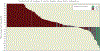
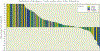
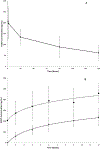
Results: From December 2007 through August 2009, 163 eligible patients from 33 institutions were enrolled. The median patient age was 31 years (range, 7-85 years). Histologic diagnoses included OS (n = 38), RMS (n = 36), SS (n = 23), and other sarcomas (n = 66). The overall objective response rate was 2.5% (95% confidence interval, 0.7%-6.2%). Partial responses were observed in 4 patients, including 2 patients with OS, 1 patient with RMS, and 1 patient with alveolar soft part sarcoma. Four additional patients (3 with RMS and 1 with myxoid liposarcoma) had a ≥ 50% decrease in tumor size that lasted for <4 weeks. The median progression-free survival was 5.7 weeks, and the median overall survival was 11 months. The most common grade 3/4 toxicities were metabolic (12%), hematologic (6%), gastrointestinal (4%), and general constitutional symptoms (8%).
Conclusions: R1507 is safe and well tolerated but has limited activity in patients with recurrent or refractory bone and soft tissue sarcomas. Additional studies to help identify the predictive factors associated with clinical benefit in selected histologies such as RMS appear to be warranted.
Keywords: IGF-1R; SARC; insulin-like growth factor; sarcoma.
© 2014 American Cancer Society.
Figures

Comment in
-
Insulin-like growth factor 1 receptor inhibitors: where do we come from? What are we? Where are we going?Cancer. 2014 Aug 15;120(16):2384-7. doi: 10.1002/cncr.28727. Epub 2014 May 2. Cancer. 2014. PMID: 24797606 No abstract available.
References
-
- Pappo AS, Devidas M, Jenkins J, et al. Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: a Pediatric Oncology Group Study. J Clin Oncol. 2005;23:4031–4038. - PubMed
-
- Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children’s Oncology Group Study D9803. J Clin Oncol. 2009;27:5182–5188. - PMC - PubMed
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, (eds). SEER Cancer Statistics Review, 1975–2010, National Cancer Institute; Bethesda, MD, HYPERLINK “/csr/1975_2010/”http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013.
-
- Stahl M, Ranft A, Paulussen M, et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer. 2011;57:549–553. - PubMed
-
- Kempf-Bielack B, Bielack SS, Jurgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005;23:559–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

