C5-alkynyl-functionalized α-L-LNA: synthesis, thermal denaturation experiments and enzymatic stability
- PMID: 24797769
- PMCID: PMC4049248
- DOI: 10.1021/jo5006153
C5-alkynyl-functionalized α-L-LNA: synthesis, thermal denaturation experiments and enzymatic stability
Abstract
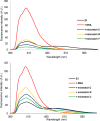
Major efforts are currently being devoted to improving the binding affinity, target specificity, and enzymatic stability of oligonucleotides used for nucleic acid targeting applications in molecular biology, biotechnology, and medicinal chemistry. One of the most popular strategies toward this end has been to introduce additional modifications to the sugar ring of affinity-inducing conformationally restricted nucleotide building blocks such as locked nucleic acid (LNA). In the preceding article in this issue, we introduced a different strategy toward this end, i.e., C5-functionalization of LNA uridines. In the present article, we extend this strategy to α-L-LNA: i.e., one of the most interesting diastereomers of LNA. α-L-LNA uridine monomers that are conjugated to small C5-alkynyl substituents induce significant improvements in target affinity, binding specificity, and enzymatic stability relative to conventional α-L-LNA. The results from the back-to-back articles therefore suggest that C5-functionalization of pyrimidines is a general and synthetically straightforward approach to modulate biophysical properties of oligonucleotides modified with LNA or other conformationally restricted monomers.
Figures




References
-
-
For recent reviews on conformationally restricted nucleotides, see e.g.:
- Herdewijn P. Chem. Biodiv. 2010, 7, 1–59. - PubMed
- Obika S.; Abdur Rahman S. M.; Fujisaka A.; Kawada Y.; Baba T.; Imanishi T. Heterocycles 2010, 81, 1347–1392.
- Prakash T. P. Chem. Biodiv. 2011, 8, 1616–1641. - PubMed
- Zhou C.; Chattopadhyaya J. Chem. Rev. 2012, 112, 3808–3832. - PubMed
-
-
-
For particularly important early examples, please see:
- Eschenmoser A. Science 1999, 284, 2118–2124. - PubMed
- Hendrix C.; Rosemeyer H.; Verheggen I.; Van Aerschot A.; Seela F.; Herdewijn P. Chem. Eur. J. 1997, 3, 110–120.
- Wang J.; Verbeure B.; Luyten I.; Lescrinier E.; Froeyen M.; Hendrix C.; Rosemeyer H.; Seela F.; Van Aerschot A.; Herdewijn P. J. Am. Chem. Soc. 2000, 122, 8595–8602.
- Bolli M.; Trafelet H. U.; Leumann C. Nucleic Acids Res. 1996, 24, 4660–4667. - PMC - PubMed
- Renneberg D.; Leumann C. J. J. Am. Chem. Soc. 2002, 124, 5993–6002. - PubMed
-
-
-
For representative recent examples, see:
- Seth P. P.; Vasquez G.; Allerson C. A.; Berdeja A.; Gaus H.; Kinberger G. A.; Prakash T. P.; Migawa M. T.; Bhat B.; Swayze E. E. J. Org. Chem. 2010, 75, 1569–1581. - PubMed
- Liu Y.; Xu J.; Karimiahmadabadi M.; Zhou C.; Chattopadhyaya J. J. Org. Chem. 2010, 75, 7112–7128. - PubMed
- Upadhayaya R.; Deshpande S. A.; Li Q.; Kardile R. A.; Sayyed A. Y.; Kshirsagar E. K.; Salunke R. V.; Dixit S. S.; Zhou C.; Foldesi A.; Chattopadhyaya J. J. Org. Chem. 2011, 76, 4408–4431. - PubMed
- Madsen A. S.; Wengel J. J. Org. Chem. 2012, 77, 3878–3886. - PubMed
- Haziri A. I.; Leumann C. J. J. Org. Chem. 2012, 77, 5861–5869. - PubMed
- Gerber A.-B.; Leumann C. J. Chem. Eur. J. 2013, 19, 6990–7006. - PubMed
- Hari Y.; Osawa T.; Kotobuki Y.; Yahara A.; Shrestha A. R.; Obika S. Bioorg. Med. Chem. 2013, 21, 4405–4412. - PubMed
- Hari Y.; Morikawa T.; Osawa T.; Obika S. Org. Lett. 2013, 15, 3702–3705. - PubMed
- Migawa M. T.; Prakash T. P.; Vasquez G.; Seth P. P.; Swayze E. E. Org. Lett. 2013, 15, 4316–4319. - PubMed
- Hanessian S.; Schroeder B. R.; Merner B. L.; Chen B.; Swayze E. E.; Seth P. P. J. Org. Chem. 2013, 78, 9051–9063. - PubMed
-
-
- Duca M.; Vekhoff P.; Oussedik K.; Halby L.; Arimondo P. B. Nucleic Acids Res. 2008, 36, 5123–5138. - PMC - PubMed
- Bennett C. F.; Swayze E. E. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259–293. - PubMed
- Østergaard M. E.; Hrdlicka P. J. Chem. Soc. Rev. 2011, 40, 5771–5788. - PMC - PubMed
- Watts J. K.; Corey D. R. J. Pathol. 2012, 226, 365–379. - PMC - PubMed
- Matsui M.; Corey D. R. Drug Discuss. Today 2012, 17, 443–450. - PMC - PubMed
- Dong H.; Lei J.; Ding L.; Wen Y.; Ju H.; Zhang X. Chem. Rev. 2013, 113, 6207–6233. - PubMed
-
- Singh S. K.; Nielsen P.; Koshkin A. A.; Wengel J. Chem. Commun. 1998, 455–456.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

