Discrepant amplification results during the development of an assay leads to reclassification of two AIDS reagent repository HIV-2 isolates as HIV-1
- PMID: 24797800
- PMCID: PMC4010467
- DOI: 10.1371/journal.pone.0096554
Discrepant amplification results during the development of an assay leads to reclassification of two AIDS reagent repository HIV-2 isolates as HIV-1
Abstract
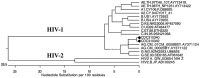
The development and verification of HIV-2 assays depends in part on the availability of well-characterized samples, including those from reagent repositories. During the development of an HIV-2 RNA quantification assay, two HIV-2 viral isolates (CDC 301340 and CDC 301342) obtained from the NIAID AIDS Reagent and Reference Repository were not detected leading to an investigation. Two HIV-2 primers/probe sets of known performance in real-time viral RNA quantification assays, targeting different regions of the virus, also failed to generate RT-PCR products for these two isolates. These isolates were tested in the HIV-1 specific COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0 (Roche Molecular Diagnostics) and were quantified at high copy number. Other HIV-2 isolates tested were not amplified in the COBAS HIV-1 TaqMan assay. Furthermore, the discrepant isolates were highly reactive in an HIV-1 p24 antigen test while the other HIV-2 isolates showed very weak, if any, cross-reactivity with the HIV-1 p24 assay. Phylogenetic tree analysis of sequences from the protease-reverse transcriptase regions of the discrepant HIV-2 isolates mapped with HIV-1 Group M, Subtype CRF02_AG confirming these isolates were of HIV-1 origin and had been misclassified as HIV-2. The use of misclassified isolates in the verification of molecular and immunological assays can lead to misinterpretation of test results, misdirection of efforts into assay redesign and increased development costs. The results of this study were shared with the NIAID AIDS Reagent Program, leading to the reclassification of the two discrepant isolates as HIV-1.
Conflict of interest statement
Figures

Similar articles
-
Evaluation of the Roche Cobas TaqMan and Abbott RealTime extraction-quantification systems for HIV-1 subtypes.J Acquir Immune Defic Syndr. 2007 Apr 15;44(5):500-5. doi: 10.1097/QAI.0b013e31803260df. J Acquir Immune Defic Syndr. 2007. PMID: 17259908
-
Comparative RNA quantification of HIV-1 group M and non-M with the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 v2.0 and Abbott Real-Time HIV-1 PCR assays.J Acquir Immune Defic Syndr. 2011 Mar 1;56(3):239-43. doi: 10.1097/QAI.0b013e3182099891. J Acquir Immune Defic Syndr. 2011. PMID: 21164353
-
Initial evaluation of the Roche COBAS TaqMan HIV-1 v2.0 assay for determining viral load in HIV-infected individuals.Antivir Ther. 2009;14(8):1189-93. doi: 10.3851/IMP1427. Antivir Ther. 2009. PMID: 20032549
-
High correlation between the Roche COBAS® AmpliPrep/COBAS® TaqMan® HIV-1, v2.0 and the Abbott m2000 RealTime HIV-1 assays for quantification of viral load in HIV-1 B and non-B subtypes.J Clin Virol. 2011 Nov;52(3):181-6. doi: 10.1016/j.jcv.2011.07.002. Epub 2011 Aug 3. J Clin Virol. 2011. PMID: 21813320
-
Verification of the Roche cobas® 6800 PCR 200 µl and 500 µl protocols for the quantification of HIV-1 RNA, HBV DNA and HCV RNA and evaluation with COBAS® Ampliprep/COBAS® TaqMan® assays.J Med Microbiol. 2018 Dec;67(12):1711-1717. doi: 10.1099/jmm.0.000838. Epub 2018 Oct 16. J Med Microbiol. 2018. PMID: 30325300
Cited by
-
Performance evaluation of a laboratory developed PCR test for quantitation of HIV-2 viral RNA.PLoS One. 2020 Feb 28;15(2):e0229424. doi: 10.1371/journal.pone.0229424. eCollection 2020. PLoS One. 2020. PMID: 32109949 Free PMC article.
References
-
- Gao F, Yue L, White AT, Pappas PG, Barchue J, et al. (1992) Human infection by genetically diverse SIVSM-related HIV-2 in west Africa. Nature 358: 495–499. - PubMed
-
- Centers for Disease Control and Prevention (2011) HIV-2 Infection Surveillance—United States, 1987-2009. MMWR Morb Mortal Wkly Rep 60: 985–988. - PubMed
-
- Ibe S, Yokomaku Y, Shiino T, Tanaka R, Hattori J, et al. (2010) HIV-2 CRF01_AB: first circulating recombinant form of HIV-2. J Acquir Immune Defic Syndr 54: 241–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

