Molecular tumor board: the University of California-San Diego Moores Cancer Center experience
- PMID: 24797821
- PMCID: PMC4041669
- DOI: 10.1634/theoncologist.2013-0405
Molecular tumor board: the University of California-San Diego Moores Cancer Center experience
Abstract
Objective: DNA sequencing tests are enabling physicians to interrogate the molecular profiles of patients' tumors, but most oncologists have not been trained in advanced genomics. We initiated a molecular tumor board to provide expert multidisciplinary input for these patients.
Materials and methods: A team that included clinicians, basic scientists, geneticists, and bioinformatics/pathway scientists with expertise in various cancer types attended. Molecular tests were performed in a Clinical Laboratory Improvement Amendments environment.
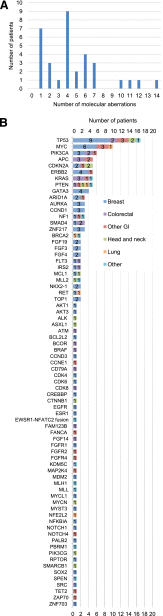
Results: Patients (n = 34, since December 2012) had received a median of three prior therapies. The median time from physician order to receipt of molecular diagnostic test results was 27 days (range: 14-77 days). Patients had a median of 4 molecular abnormalities (range: 1-14 abnormalities) found by next-generation sequencing (182- or 236-gene panels). Seventy-four genes were involved, with 123 distinct abnormalities. Importantly, no two patients had the same aberrations, and 107 distinct abnormalities were seen only once. Among the 11 evaluable patients whose treatment had been informed by molecular diagnostics, 3 achieved partial responses (progression-free survival of 3.4 months, ≥6.5 months, and 7.6 months). The most common reasons for being unable to act on the molecular diagnostic results were that patients were ineligible for or could not travel to an appropriately targeted clinical trial and/or that insurance would not cover the cognate agents.
Conclusion: Genomic sequencing is revealing complex molecular profiles that differ by patient. Multidisciplinary molecular tumor boards may help optimize management. Barriers to personalized therapy include access to appropriately targeted drugs.
Keywords: Cancer; Molecular profile; Molecular tumor board; Mutation; Personalized.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Dancey JE, Bedard PL, Onetto N, et al. The genetic basis for cancer treatment decisions. Cell. 2012;148:409–420. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. - PubMed
-
- Wong KM, Hudson TJ, McPherson JD. Unraveling the genetics of cancer: Genome sequencing and beyond. Annu Rev Genomics Hum Genet. 2011;12:407–430. - PubMed
-
- Barrett JC, Frigault MM, Hollingsworth S, et al. Are companion diagnostics useful? Clin Chem. 2013;59:198–201. - PubMed
-
- Montemurro F, Valabrega G, Aglietta M. Trastuzumab treatment in breast cancer. N Engl J Med. 2006;354:2186–author reply 2186. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

