Enabling a genetically informed approach to cancer medicine: a retrospective evaluation of the impact of comprehensive tumor profiling using a targeted next-generation sequencing panel
- PMID: 24797823
- PMCID: PMC4041676
- DOI: 10.1634/theoncologist.2014-0011
Enabling a genetically informed approach to cancer medicine: a retrospective evaluation of the impact of comprehensive tumor profiling using a targeted next-generation sequencing panel
Abstract
Background: Oncogenic genetic alterations "drive" neoplastic cell proliferation. Small molecule inhibitors and antibodies are being developed that target an increasing number of these altered gene products. Next-generation sequencing (NGS) is a powerful tool to identify tumor-specific genetic changes. To determine the clinical impact of extensive genetic analysis, we reviewed our experience using a targeted NGS platform (FoundationOne) in advanced cancer patients.
Patients and methods: We retrospectively assessed demographics, NGS results, and therapies received for patients undergoing targeted NGS (exonic sequencing of 236 genes and selective intronic sequencing from 19 genes) between April 2012 and August 2013. Coprimary endpoints were the percentage of patients with targeted therapy options uncovered by mutational profiling and the percentage who received genotype-directed therapy.
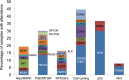
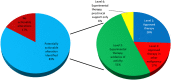
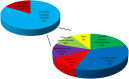
Results: Samples from 103 patients were tested, most frequently breast carcinoma (26%), head and neck cancers (23%), and melanoma (10%). Most patients (83%) were found to harbor potentially actionable genetic alterations, involving cell-cycle regulation (44%), phosphatidylinositol 3-kinase-AKT (31%), and mitogen-activated protein kinase (19%) pathways. With median follow-up of 4.1 months, 21% received genotype-directed treatments, most in clinical trials (61%), leading to significant benefit in several cases. The most common reasons for not receiving genotype-directed therapy were selection of standard therapy (35%) and clinical deterioration (13%).
Conclusion: Mutational profiling using a targeted NGS panel identified potentially actionable alterations in a majority of advanced cancer patients. The assay identified additional therapeutic options and facilitated clinical trial enrollment. As time progresses, NGS results will be used to guide therapy in an increasing proportion of patients.
Keywords: Cancer; Genotype; Molecular targeted therapy; Mutation; Next-generation sequencing; Precision medicine.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Bos JL. ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. - PubMed
-
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. - PubMed
-
- Nakahara M, Isozaki K, Hirota S, et al. A novel gain-of-function mutation of c-kit gene in gastrointestinal stromal tumors. Gastroenterology. 1998;115:1090–1095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

