Type IV pilus assembly proficiency and dynamics influence pilin subunit phospho-form macro- and microheterogeneity in Neisseria gonorrhoeae
- PMID: 24797914
- PMCID: PMC4010543
- DOI: 10.1371/journal.pone.0096419
Type IV pilus assembly proficiency and dynamics influence pilin subunit phospho-form macro- and microheterogeneity in Neisseria gonorrhoeae
Abstract
The PilE pilin subunit protein of the gonococcal Type IV pilus (Tfp) colonization factor undergoes multisite, covalent modification with the zwitterionic phospho-form modification phosphoethanolamine (PE). In a mutant lacking the pilin-like PilV protein however, PilE is modified with a mixture of PE and phosphocholine (PC). Moreover, intrastrain variation of PilE PC modification levels have been observed in backgrounds that constitutively express PptA (the protein phospho-form transferase A) required for both PE and PC modification. The molecular basis underlying phospho-form microheterogeneity in these instances remains poorly defined. Here, we examined the effects of mutations at numerous loci that disrupt or perturb Tfp assembly and observed that these mutants phenocopy the pilV mutant vis a vis phospho-form modification status. Thus, PC modification appears to be directly or indirectly responsive to the efficacy of pilin subunit interactions. Despite the complexity of contributing factors identified here, the data favor a model in which increased retention in the inner membrane may act as a key signal in altering phospho-form modification. These results also provide an alternative explanation for the variation in PilE PC levels observed previously and that has been assumed to be due to phase variation of pptA. Moreover, mass spectrometry revealed evidence for mono- and di-methylated forms of PE attached to PilE in mutants deficient in pilus assembly, directly implicating a methyltransferase-based pathway for PC synthesis in N. gonorrhoeae.
Conflict of interest statement
Figures
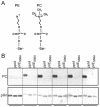

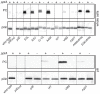
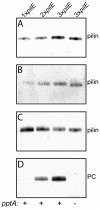
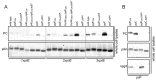

Similar articles
-
Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases.J Biol Chem. 2006 Sep 22;281(38):27712-23. doi: 10.1074/jbc.M604324200. Epub 2006 Jul 5. J Biol Chem. 2006. PMID: 16825186
-
Unique modifications with phosphocholine and phosphoethanolamine define alternate antigenic forms of Neisseria gonorrhoeae type IV pili.Proc Natl Acad Sci U S A. 2004 Jul 20;101(29):10798-803. doi: 10.1073/pnas.0402397101. Epub 2004 Jul 12. Proc Natl Acad Sci U S A. 2004. PMID: 15249686 Free PMC article.
-
Novel protein substrates of the phospho-form modification system in Neisseria gonorrhoeae and their connection to O-linked protein glycosylation.Infect Immun. 2012 Jan;80(1):22-30. doi: 10.1128/IAI.05920-11. Epub 2011 Nov 14. Infect Immun. 2012. PMID: 22083701 Free PMC article.
-
Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae--a review.Gene. 1997 Jun 11;192(1):125-34. doi: 10.1016/s0378-1119(97)00038-3. Gene. 1997. PMID: 9224882 Review.
-
The role of pilin glycan in neisserial pathogenesis.Mol Cell Biochem. 2003 Nov;253(1-2):179-90. doi: 10.1023/a:1026058311857. Mol Cell Biochem. 2003. PMID: 14619968 Review.
Cited by
-
The biosynthesis and role of phosphorylcholine in pathogenic and nonpathogenic bacteria.Trends Microbiol. 2023 Jul;31(7):692-706. doi: 10.1016/j.tim.2023.01.006. Epub 2023 Feb 28. Trends Microbiol. 2023. PMID: 36863982 Free PMC article. Review.
-
Analysis of Bacterial Phosphorylcholine-Related Genes Reveals an Association between Type-Specific Biosynthesis Pathways and Biomolecules Targeted for Phosphorylcholine Modification.Microbiol Spectr. 2023 Aug 17;11(4):e0158323. doi: 10.1128/spectrum.01583-23. Epub 2023 Jul 12. Microbiol Spectr. 2023. PMID: 37436144 Free PMC article.
References
-
- Mackinnon FG, Cox AD, Plested JS, Tang CM, Makepeace K, et al. (2002) Identification of a gene (lpt-3) required for the addition of phosphoethanolamine to the lipopolysaccharide inner core of Neisseria meningitidis and its role in mediating susceptibility to bactericidal killing and opsonophagocytosis. Mol Microbiol 43: 931–943. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

