Nutritional and metabolic requirements for the infection of HeLa cells by Salmonella enterica serovar Typhimurium
- PMID: 24797930
- PMCID: PMC4010460
- DOI: 10.1371/journal.pone.0096266
Nutritional and metabolic requirements for the infection of HeLa cells by Salmonella enterica serovar Typhimurium
Abstract
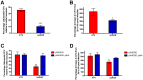
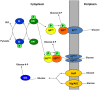
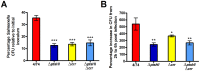
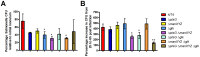
Salmonella is the causative agent of a spectrum of human and animal diseases ranging from gastroenteritis to typhoid fever. It is a food--and water--borne pathogen and infects via ingestion followed by invasion of intestinal epithelial cells and phagocytic cells. In this study we employed a mutational approach to define the nutrients and metabolic pathways required by Salmonella enterica serovar Typhimurium during infection of a human epithelial cell line (HeLa). We deleted the key glycolytic genes, pfkA and pfkB to show that S. Typhimurium utilizes glycolysis for replication within HeLa cells; however, glycolysis was not absolutely essential for intracellular replication. Using S. Typhimurium strains deleted for genes encoding components of the phosphotransferase system and glucose transport, we show that glucose is a major substrate required for the intracellular replication of S. Typhimurium in HeLa cells. We also deleted genes encoding enzymes involved in the utilization of gluconeogenic substrates and the glyoxylate shunt and show that neither of these pathways were required for intracellular replication of S. Typhimurium within HeLa cells.
Conflict of interest statement
Figures

Similar articles
-
Polyamines are required for virulence in Salmonella enterica serovar Typhimurium.PLoS One. 2012;7(4):e36149. doi: 10.1371/journal.pone.0036149. Epub 2012 Apr 30. PLoS One. 2012. PMID: 22558361 Free PMC article.
-
A comprehensive study of the contribution of Salmonella enterica serovar Typhimurium SPI2 effectors to bacterial colonization, survival, and replication in typhoid fever, macrophage, and epithelial cell infection models.Virulence. 2011 May-Jun;2(3):208-16. doi: 10.4161/viru.2.3.15894. Epub 2011 May 1. Virulence. 2011. PMID: 21540636 Free PMC article.
-
Glucose and glycolysis are required for the successful infection of macrophages and mice by Salmonella enterica serovar typhimurium.Infect Immun. 2009 Jul;77(7):3117-26. doi: 10.1128/IAI.00093-09. Epub 2009 Apr 20. Infect Immun. 2009. PMID: 19380470 Free PMC article.
-
The challenge of relating gene expression to the virulence of Salmonella enterica serovar Typhimurium.Curr Opin Biotechnol. 2011 Apr;22(2):200-10. doi: 10.1016/j.copbio.2011.02.007. Epub 2011 Mar 22. Curr Opin Biotechnol. 2011. PMID: 21388802 Review.
-
Cross-Talk Between the Intestinal Epithelium and Salmonella Typhimurium.Front Microbiol. 2022 Jun 6;13:906238. doi: 10.3389/fmicb.2022.906238. eCollection 2022. Front Microbiol. 2022. PMID: 35733975 Free PMC article. Review.
Cited by
-
Intracellular Salmonella Paratyphi A is motile and differs in the expression of flagella-chemotaxis, SPI-1 and carbon utilization pathways in comparison to intracellular S. Typhimurium.PLoS Pathog. 2022 Apr 5;18(4):e1010425. doi: 10.1371/journal.ppat.1010425. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35381053 Free PMC article.
-
Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death.Elife. 2016 Mar 24;5:e13663. doi: 10.7554/eLife.13663. Elife. 2016. PMID: 27011353 Free PMC article.
-
Identification of common highly expressed genes of Salmonella Enteritidis by in silico prediction of gene expression and in vitro transcriptomic analysis.Poult Sci. 2019 Jul 1;98(7):2948-2963. doi: 10.3382/ps/pez119. Poult Sci. 2019. PMID: 30953073 Free PMC article.
-
Dual transcriptome based reconstruction of Salmonella-human integrated metabolic network to screen potential drug targets.PLoS One. 2022 May 24;17(5):e0268889. doi: 10.1371/journal.pone.0268889. eCollection 2022. PLoS One. 2022. PMID: 35609089 Free PMC article.
-
Cyclic-di-GMP regulation promotes survival of a slow-replicating subpopulation of intracellular Salmonella Typhimurium.Proc Natl Acad Sci U S A. 2019 Mar 26;116(13):6335-6340. doi: 10.1073/pnas.1901051116. Epub 2019 Mar 12. Proc Natl Acad Sci U S A. 2019. PMID: 30862737 Free PMC article.
References
-
- Garcia-del Portillo F (2001) Salmonella intracellular proliferation: where, when and how? Microbes Infect 3: 1305–1311. - PubMed
-
- Abrahams GL, Hensel M (2006) Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol 8: 728–737. - PubMed
-
- Haraga A, Ohlson MB, Miller SI (2008) Salmonellae interplay with host cells. Nat Rev Microbiol 6: 53–66. - PubMed
-
- Brumell JH, Steele-Mortimer O, Finlay BB (1999) Bacterial invasion: Force feeding by Salmonella . Curr Biol 9: R277–280. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

